matter & views of chem
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
atom?
a particle that is used as a building block for matter. examples: boron, hydrogen, oxygen, etc.
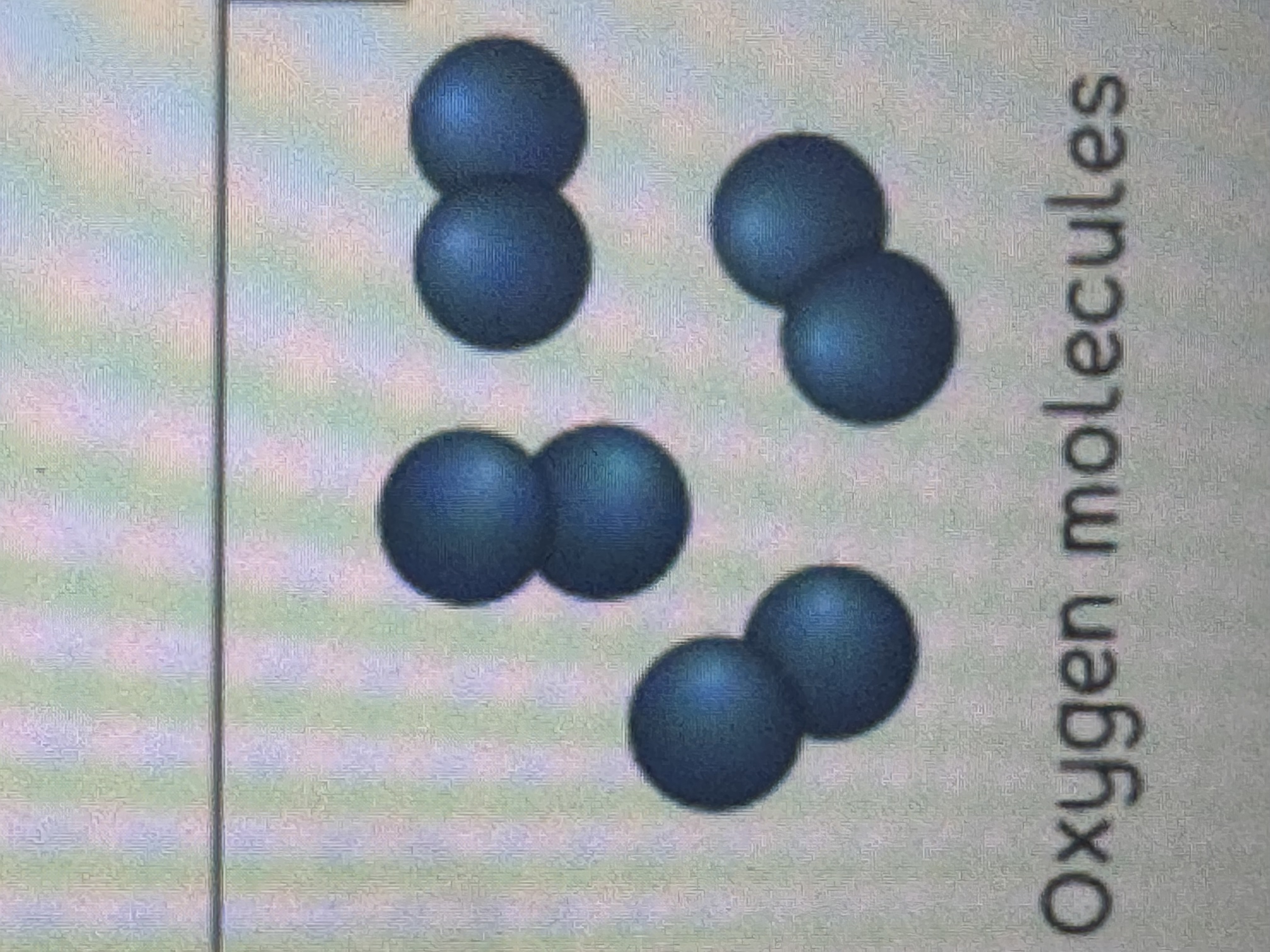
element?
two atoms stuck together, two atoms boned as a unit. 1 SOLID COLOR OF AN ATOM. examples: hydrogen, boron, helium, etc.
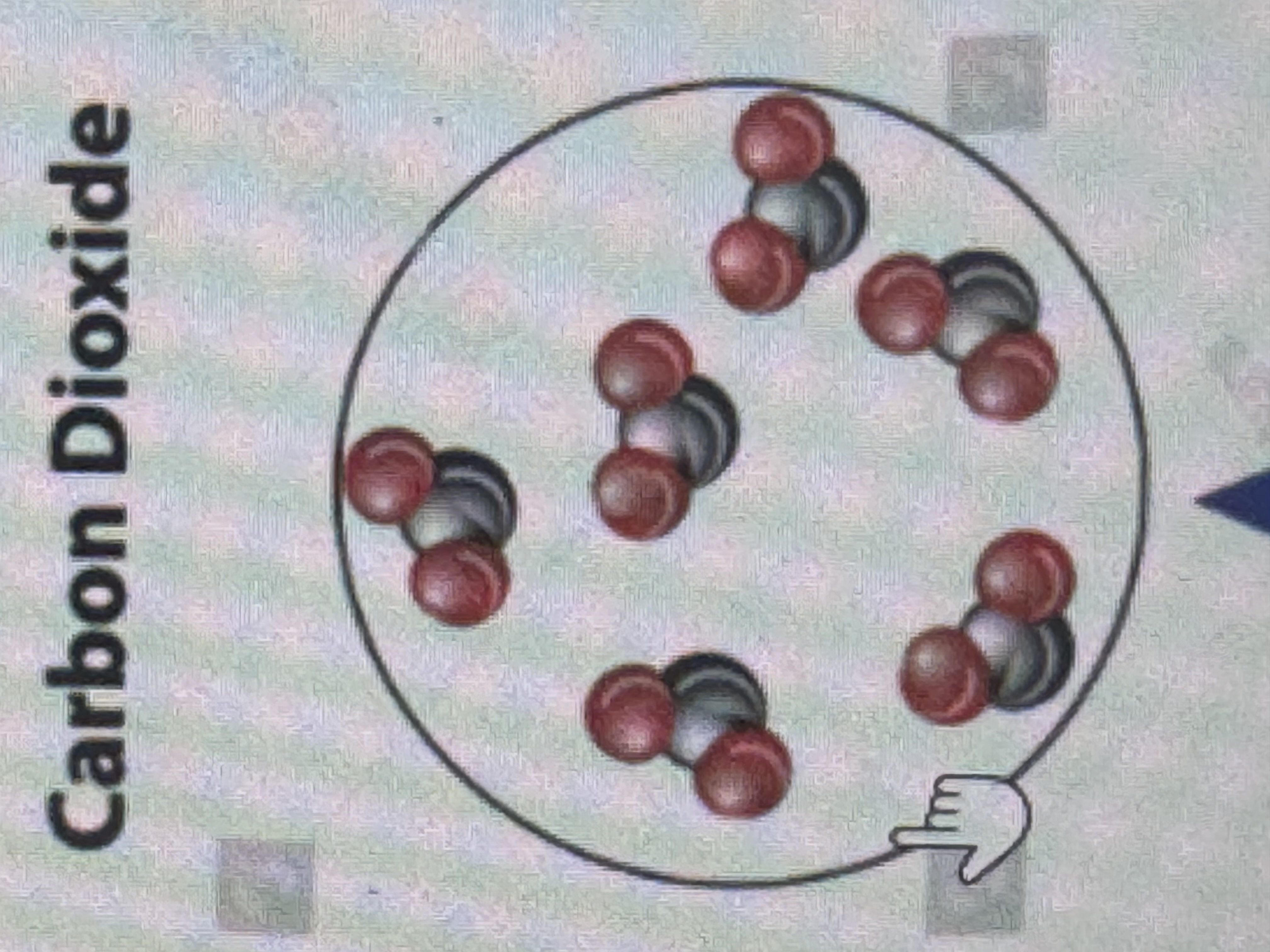
compound?
two or more elements combined, 2 DIFFERENT COLOR OF ATOMS. examples: water, carbon dioxide, glucose, etc.
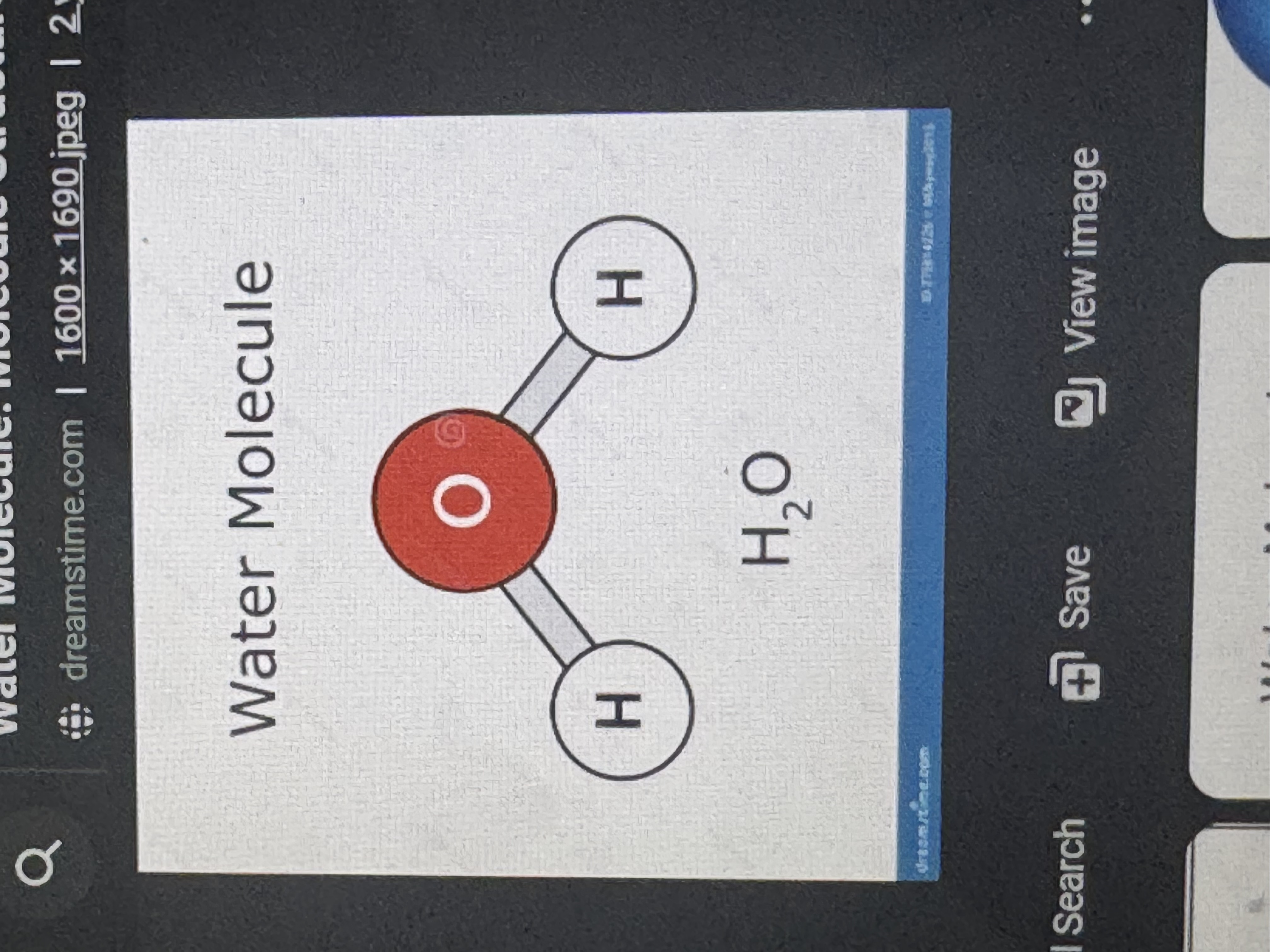
molecule ?
two or more atoms joined chemically in a specific structure. examples: h2o
macroscopic?
what the human eye sees.

symbolic?
a short notation to represent elements.

particulate?
a representation of each atom, where different atoms types are shown w a unique symbol.
heterogeneous mixture?
a non consistent mixture. example: water and oil or cookies. (pieces of each atom can be separated easily.)

homogenous mixture?
a consistent and uniform mixture. example: flour & water.

pure substance?
a substance made up of only one component and contains individual atoms joined together.

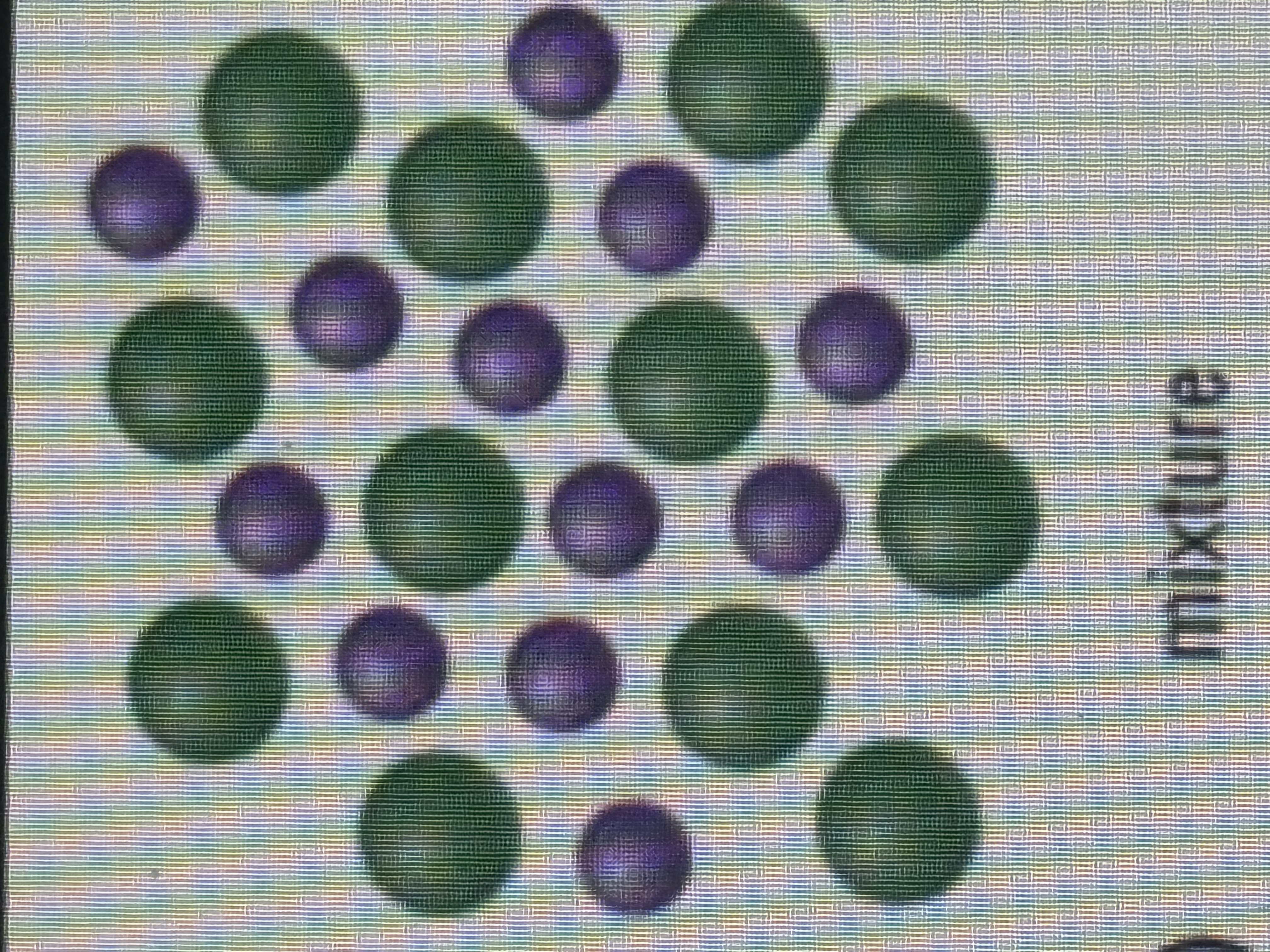
mixture?
a substance composed of two or more components which can differ from one another.