Chapter 16 | pH and Buffers
0.0(0)
Card Sorting
1/6
There's no tags or description
Looks like no tags are added yet.
Last updated 2:23 AM on 8/16/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
1
New cards
Buffer
Any solution that maintains an approximately constant pH despite small additions of acid and base
2
New cards
Buffered solutions
Can be made from:
Weak acid & its conjugate base (strong base) or
Weak base and strong acid
Ratio has to be close to 1:1
3
New cards
Henderson-Hasselbalch equation
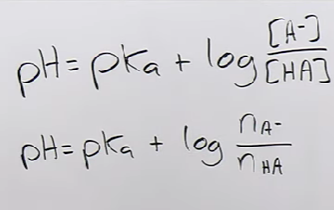
4
New cards
Strong acid & base titrations
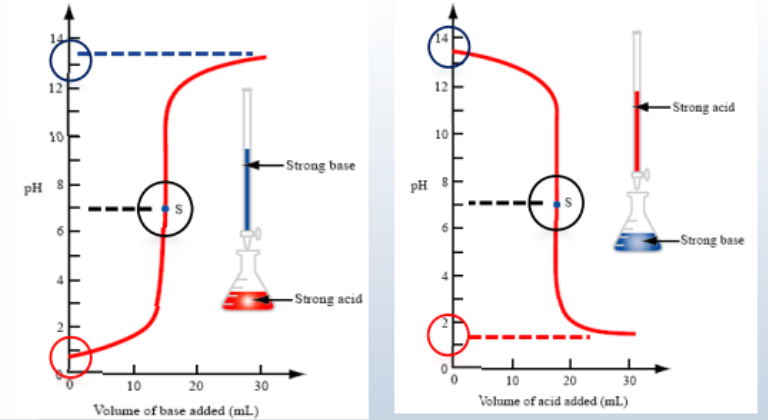
5
New cards
Weak acid - strong base titration
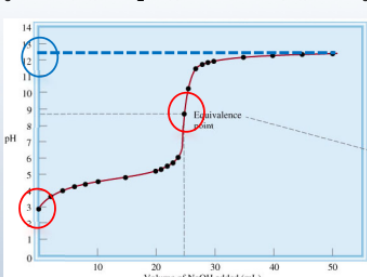
6
New cards
Strong acid - weak base titration
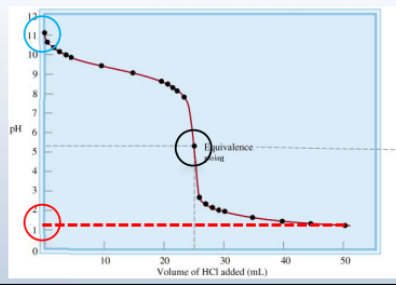
7
New cards
Common ion effect
Adding a compound with an ion that's already in a solution reduces the solubility of another compound in that solution