The d- and f - Block Elements
1/43
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
44 Terms
d-Block
Elements whose last filled electron enters into a d-orbital in a pen-ultimate shell of the atom are called d-block elements
The d-block of the periodic table contains the elements of the groups 3-12
d-block elements can be classified into four series:
3d series (first transition series):
21Sc -> 30Zn (10 elements)
4d series (second transition series):
39Y -> 48Cd (10 elements)
5d series (third transition series):
57La -> 80Hg (10 elements)
6d series (fourth transition series):
89Ac -> 112Cn (10 elements)
d-block elements are
Metallic elements; have a higher metallic character than s-block elements
Metals:
Have a very hard crystalline structure
High melting and boiling point
Mostly paramagnetic
Electro +ve with low ionisation energy
Show variable oxidation state
Show the highest catalytic properties and can form complex compounds easily
Electronic Configurations of the d-Block Elements
General config: (n-1)d1-10ns1-2
Special Cases:
Pd config: 4d105so
Cr config: [Ar]3d54s1 and Cu config: [Ar]3d104s1
This is because:
Half and completely-filled sets of orbitals are relatively more stable
The energy gap between the two sets (3d and 4s) of orbitals is small enough to prevent electrons from entering the 3d orbitals
d-block elements as Transition elements
Elements with partially or incompletely filled d-orbital in their ground state or any one of its stable oxidation state
Zn, Cd and Hg are not considered transition elements since they have completely filled d-orbital in their ground and stable oxidation state
Their ground state config looks like this: (n-1)d10ns2
Even in their +2 common oxidation state, they are completely filled
Cu and Ag have completely filled d-orbitals in their ground state but are transition elements in their stable oxidation state of +2 where they have a d9 configuration
Identifying Group and Period of a d-block Element based on Config
For any d-block element, (n-1)dxnsy
group = x+y
period = n
Atomic Radii
The equilibrium distance between the centre of the nucleus and the outermost energy level
In periods: decreases
due to an increase in nuclear charge
In groups: increases
Towards the end of a period, the elements show a very small difference in atomic radii and show a gradual increase in size due electron pairing effect of d-orbitals and an increased shielding effect which counterbalances the increase in nuclear charge
Effective nuclear charge Z* = Z (nuclear charge) - S (shielding effect) see note for example
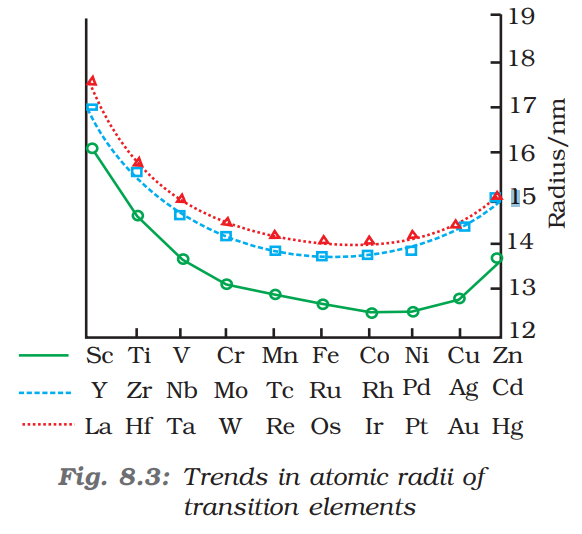
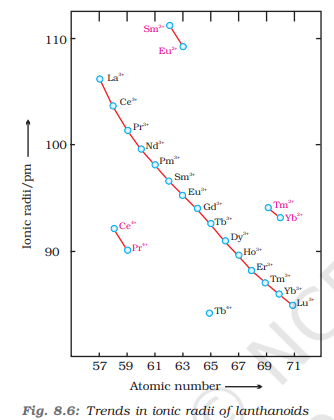
Lanthanoid Contraction
Among d-block elements 4d and 5d series elements show similar atomic radii and similar physical properties due to lanthanoid contraction
Eg: Zr and Hf have similar atomic radii and similar physical properties
A regular decrease in atomic radii of lanthanoid series elements with increase in atomic number is known as lanthanoid contraction
This is due to poor shielding of 4f electrons
Ionic Radii
Ionic size decreases with increase in oxidation state
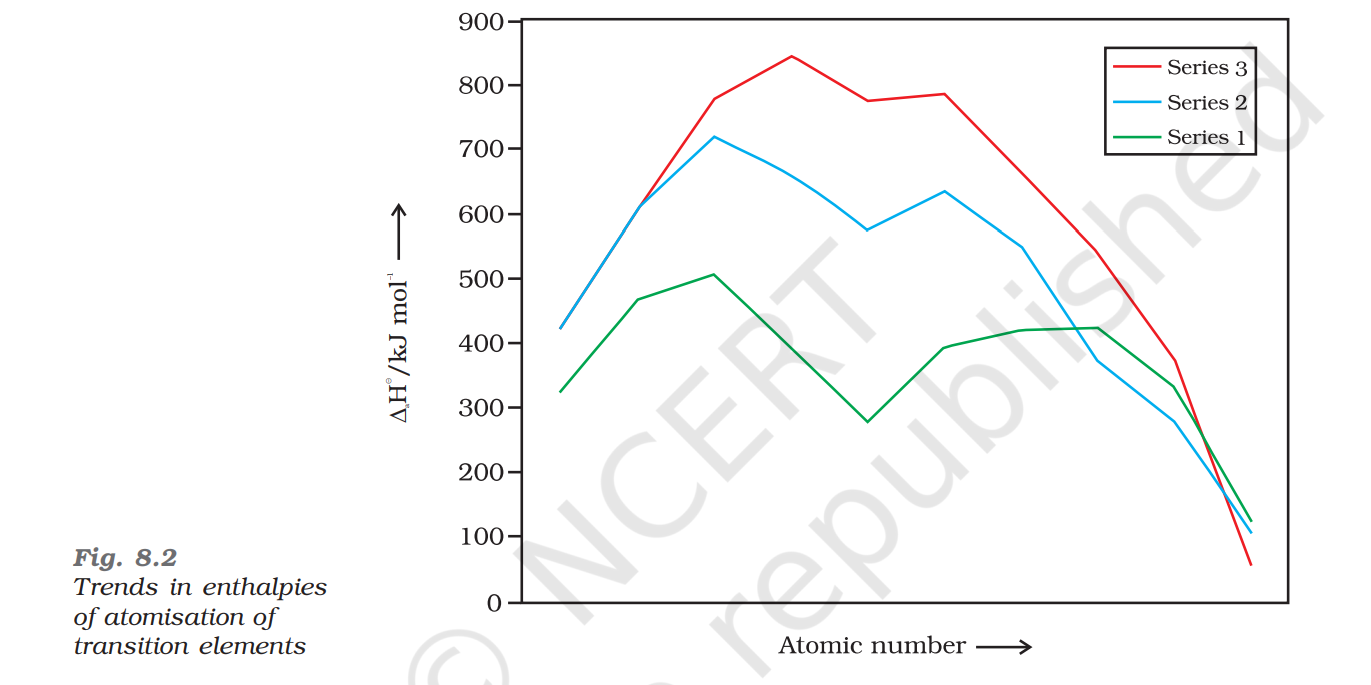
Enthalpy of Atomisation
The energy required to form one mole of atoms from a molecule in standard elemental form
Depends on:
Strength of metallic bond which in turn depends on,
Number of unpaired electrons
EoA ∝ Metallic Bond ∝ Number of unpaired electrons
As we move across a period, the enthalpy of atomisation increases and then decreases
In the 3d series, Zinc has the lowest enthalpy of atomisation since it has a filled d10 configuration and hence has a lower metallic bond strength
Personal Note: the graph is not in the order of each series
Ionisation Enthalpy/Energy/Potential
The energy required to remove the most loosely bound electron from the outermost energy level of an isolated, neutral and gaseous atom.
Factors affecting I.E:
I.E ∝ 1/Atomic Size
I.E ∝ Nuclear Charge
I.E ∝ 1/Shielding Effect
I.E ∝ 1/Complexity of Shape
I.E ∝ Stability of Configuration
Across periods: increases
Across groups: decreases
For a given atom, the first ionisation energy will be less than the second, less than the third and so on
I.E.1 < I.E.2 < I.E.3 < ...
When an electron is removed from a stable configuration, greater I.E. is used
When an electron is removed to attain a stable configuration, less I.E. is used
In d-block elements, the possible stable configurations are:
d0 → Noble gas config
d3 → Half filled t2g config
d5 → Half filled d-config
d6 → Completely filled t2g config
d10 → Completely filled d-config
In 3d series, element with
lowest I.E → Sc
highest I.E → Zn
see note for examples of higher I.E2, I.E3, etc.
Melting and Boiling Points
The melting and boiling points of an element depend on the strength of the metallic bond in its metallic crystal lattices
Across periods: increases then decreases
Across groups: generally increases
Mn has lesser M.P and B.P when compared to its neighbours since it has a half-filled d5 configuration which is not involved in the formation of a metallic bond. Due to the weak metallic bond, it has a weak M.P and B.P
Zn has a low M.P and B.P among 3d series elements because it has a stable completely filled d10 configuration which is not involved in the formation of a metallic bond. Due to the weak metallic bond, it has a weak M.P and B.P
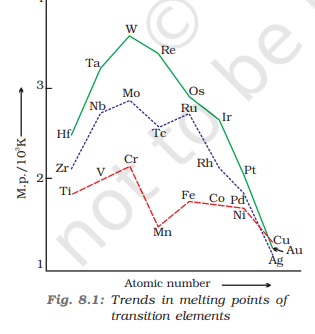
Which element has the highest M.P in 3d series and entire d-block?
In the 3d series, Cr has the highest melting point because it has the highest number of unpaired electrons (3d54s1 config) and therefore has a greater metallic bond strength
Tungsten (W) has highest M.P in d-block
Oxidation State
d-block elements can show variable oxidation state
due to the small energy difference between ns and (n-1)d orbitals
Most common O.S is +2
Across periods: increases then decreases
Can form compounds involving the valence electrons as well as the inner d-orbitals
The acidic character of compounds increases with an increase in O.S
Mn can combine with 7 F in its highest O.S but can combine only with 4 O in higher O.S
O.S ∝ Covalent character
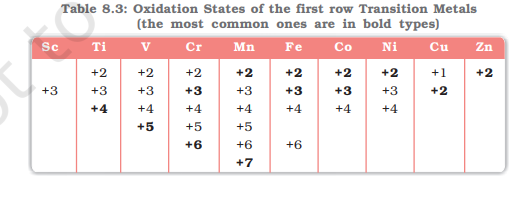
Sc does now show +2 O.S
Sc only has +3 O.S
Sc does not show variable O.S
Why?
Sc has a 3d14s2 configuration and by losing 3 electrons it gains noble gas configuration
Mn has the maximum number of O.S. Why?
Mn has a 3d54s2 configuration. Hence it can show an oxidation state ranging from +2 to +7
Zn has only +2 O.S. Why?
Zn has 3d104s2 and by losing 2 electrons it gains completely filled d10 configuration
Cu is the only element in 3d series with +1 O.S. Why?
Cu has a configuration [Ar]3d104s1 and by losing 1 electron it gains completely filled d10 configuration
Elements of d-block preferentially form fluorides and oxides in their higher O.S. Why?
Due to small size and high electro-negativity of O and F
Special Elements
Highest O.S: Osmium (OS) +8 O.S
Maximum number of O.S: Mn (+2 - +7)
Electrode Potential
Across periods: increases due to an increase in nuclear charge and decrease in atomic size
Atomisation enthalpy increases, ionisation enthalpy increases, hydration enthalpy decreases
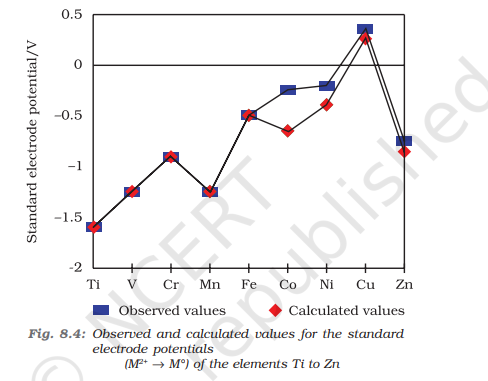
Cu has a +ve electrode potential. Why?
The high transformation energy from Cu(s) to Cu2+(aq) is not balanced by hydration enthalpy

The electrode potential of Nickel is more negative than expected. Why?
Nickel has relatively higher hydration enthalpy or more negative hydration enthalpy
Mn has a more negative electrode potential value than expected. Why?
Due to presence of stable d-5 configuration after losing electrons (due to very low IE2)
Zn has more negative electrode potential value than expected. Why?
Due to presence of a completely filled stable d10 configuration after losing 2 electrons
Oxidising and Reducing Power
Oxidation /Reducing Agent - Loses/Removes Electron
Oxidising Agent/Reduction - Gains Electron
examples in note
d-block elements can act as both oxidising and reducing agents in their combined forms
Any species which gains electrons to attain a stable configuration is a very good oxidising agent. Eg: Mn3+
Any species which loses electrons to attain a stable configuration is a very good reducing agent. Eg: Fe2+
Magnetic Properties
Mostly paramagnetic due to the presence of unpaired electron
Only Sc and Zn are diamagnetic due to no unpaired electrons
Magnetic moment, μ = √n(n+2) B.M
n => number of unpaired electrons
B.M => Bohrn magneton
n=1, 1.7 B.M
n=2, 2.8 B.M
n=3, 3.8 B.M
n=4, 4.9 B.M
n=5, 5.9 B.M
Formation of Ions
Most of the d-block elements form coloured ions in their compounds due to the presence of unpaired electrons as they take part in d-d transition
Few ions of d-block are colourless due to the absence of unpaired electrons as no d-d transition takes place. Eg: Cu+, Sc3+, Ti4+, Zn2+
Red-orange colours are typically observed with ions that have a higher number of unpaired electrons.
Blue and violet colours are typically observed with ions that have a lower number of unpaired electrons
black or white is considered colourless
Catalytic Properties
d-block elements can act as a very good catalyst. Due to presence of more vacant d-orbitals, they show variable oxidation state and can form intermediate complex easily in turn reducing the activation energy of the reaction
Formation of Interstitial Compounds
Interstitial compounds are those which are formed when small atoms like H, C or N are trapped inside the crystal lattices of metals.
They are usually non-stoichiometric and are neither typically ionic nor covalent,
The principal physical and chemical characteristics of these compounds are as follows:
They have high melting points, higher than those of pure metals.
They are very hard, some borides approach diamonds in hardness
They retain metallic conductivity
They are chemically inert
Alloy Formation
A homogenous mixture of a metal-metal/metal-non-metal
d-block elements can form alloys easily due to similar atomic radii
These elements can replace one another in metallic crystal lattices due to similar size
Eg: Bronze (Copper and Tin), Brass (Copper and Zinc)
Oxides and Oxoanions of Metals
not important
High oxidation states oxide are acidic
Ex: CrO3, Mn2O7
remember as Higher - HCl (Acidic)
Lower oxidation state oxides are basic
Ex: MnO, Mn2O3, CrO
Scandium does not form metal oxide
Important Compounds of d-block
Potassium dichromate (K2Cr2O7)
Potassium permanganate (KMnO4)
Preparation of Potassium dichromate
chromite ore (Cr2O42-) → chromate (CrO42-) → dichromate (Cr2O72-)
Convert chromite ore to sodium chromate (Oxidation of ore in sodium carbonate)
4FeCr2O4 + 8Na2CO3 + 7O2 → 8Na2CrO4 + 2Fe2O3 + 8CO2
Sodium chromate is filtered since it has impurities
Convert sodium chromate to sodium dichromate (Acidification by H2SO4)
2Na2CrO4 + H2SO4 → Na2Cr2O7 + Na2SO4 + H2O
Convert sodium dichromate to potassium dichromate (Displace with KCl)
Na2Cr2O7 + 2KCl → K2Cr2O7 + 2NaCl
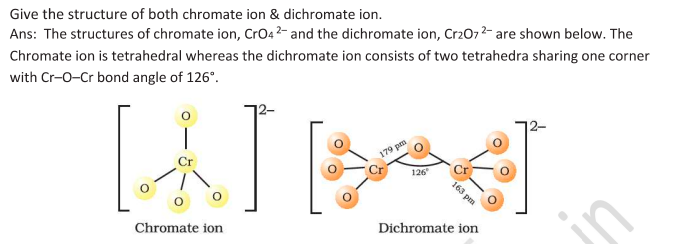
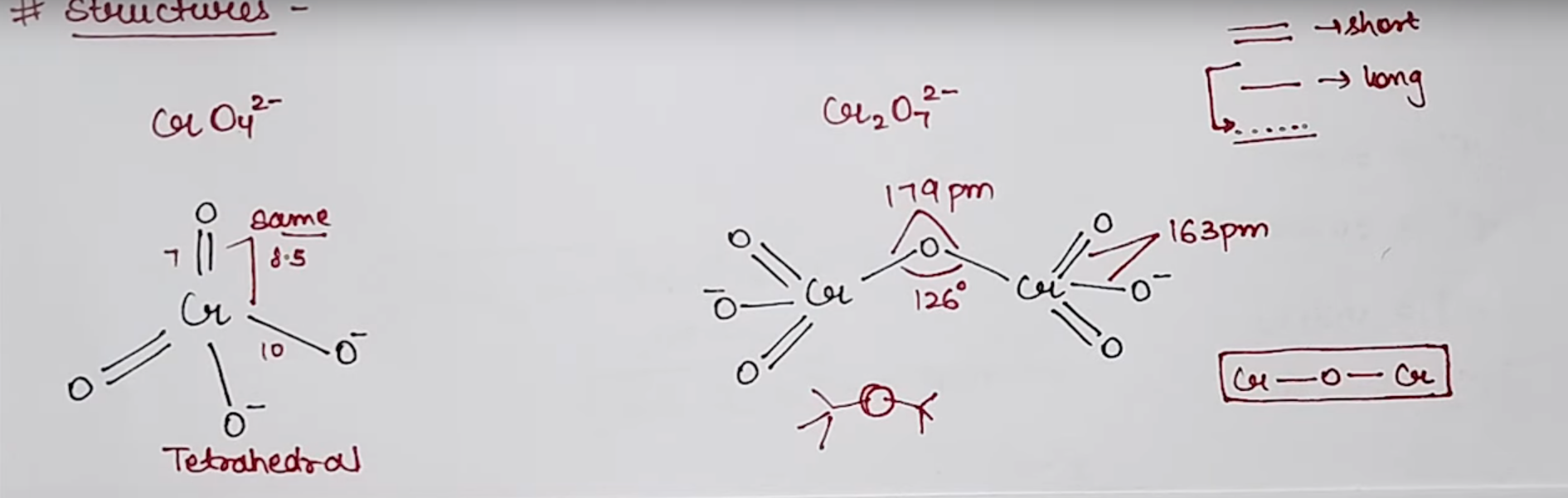
Structures of Chromate and Dichromate
Ditetrahedral Geometry
Red orange crystal
Properties of Potassium Dichromate
Very strong oxidising agent in acidic medium
It can oxidise iodide
I⁻ → I₂
Fe²⁺ → Fe3+
Sn²⁺ → Sn⁴⁺
S2- → S
NO2- → NO3-
SO32- → SO42-
Can form interconvertible aqueous solutions based on the pH of the solution
In acidic medium:
CrO42- (yellow) → [H+] Cr2O72- (orange)
Chromate ion is more stable in basic medium. In acidic medium chromate becomes dichromate.
In basic medium:
Cr2O72- (red-orange) → [OH-] CrO42- (yellow)
Dichromate ion is more stable in acidic medium. In basic medium dichromate becomes chromate.
Has a greater solubility, used in a primary standard solution in volumetric analysis
It is an industrially used chemical oxidant used in the preparation of azo compounds and in the leather industries
Used in chromyl chloride test for confirmation of chloride ion in inorganic compounds
Preparation of Potassium Permanganate
Pyrolusite ore to Potassium Manganate
2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
Potassium Manganate to Potassium Permanganate (Disproportionation reaction)
3K2MnO4 + 4H+ → 2KMnO4 + MnO2 + 2H2O

Properties of Potassium Permanganate
Dark purple crystal and comparatively less soluble in water
It is purple due to the metal-ligand electron transition
At higher temperatures (513K), it decomposes to produce potassium manganate and magnesium dioxide
2KMnO4 → [Δ, 513K] K2MnO4 + MnO2 + O2
It is diamagnetic due to the absence of an unpaired electron
It is a very strong oxidising agent in acidic as well as alkaline medium. It can oxidise:
In acidic:
I⁻ → I₂ (X⁻ → X₂)
Fe²⁺ → Fe3+
S2- → S
NO2- → NO3-
SO32- → SO42-
C2O42- → CO2
In basic:
I⁻ → IO3- (X⁻ → XO3-)
Mn²⁺ → MnO2
S2O32- → SO42-
It is used as an oxidising agent in the acidic, basic and neutral mediums in laboratory & industry.
For volumetric estimation of ferrous salts, oxalates and other reducing agents.
As disinfectant for water.
In dry cells.
It is used for bleaching wool, cotton, silk, and other textile fibres and decolourising oils.
f-Block
Elements whose last electron enters the outermost f-orbital
Two rows of high-density elements are embedded within the third group and placed at the bottom of the periodic table. These are called f-block elements.
General config (n−2)f1−14(n−1)d0−1ns2
Lanthanoids:
58Ce → 71Lu (14 elements)
Actinoids:
90Th → 103Lr (14 elements)
Consequences of Lanthanoid Contraction
4d and 5d series show similar atomic radii and physical properties
It’s difficult to separate lanthanoids
The basic character of lanthanide hydroxides decreases from lanthanum (La) to lutetium (Lu)
Oxidation State of Lanthanoids
Common O.S → +3
Ce → +4
Eu, Yb → +2
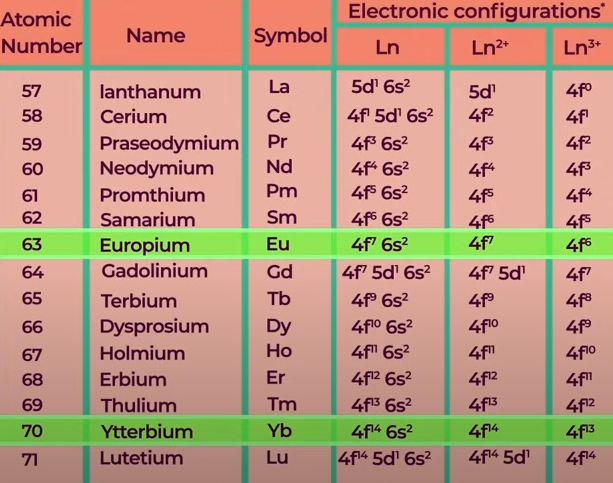
General Characteristics of Lanthanoids
Silvery white soft metals
Tarnish rapidly in air
Hardness increases with increasing atomic number, Samarium being steel hard.
Melting points range between 1000 to 1200 K but Samarium melts at 1623 K.
Typical metallic structure
Good conductors of heat and electricity
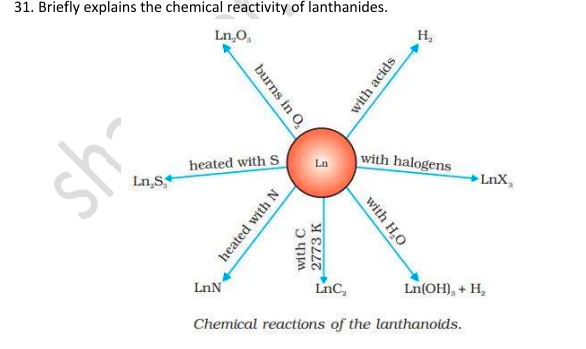
Chemical Reactions of Lanthanoids
Actinoids
Radioactive elements
Earlier members have relatively long half-lives, the latter ones have half-life values ranging from a day to 3 minutes
Actinoid contraction is the overall decrease in atomic and ionic radii with increase in atomic number of actinoids due to poor shielding effect of f-electrons
Show a greater range of oxidation states due to very less energy difference between 5f, 6d and 7s orbitals
Most common oxidation state is +3
Highly reactive metals especially when finely divided
Differentiate between Lanthanoids and Actinoids
Lanthanoids:
Except for Pm, lanthanoids are non-radioactive
Common O.S is +3, they can also show +2 and +4
Don’t form oxo cations
Have less tendency to form complexes
Show regular decrease in atomic radii due to lanthanoid contraction
Actinoids:
All actinoids are radioactive
Common O.S is +3 but can show various O.S
Form oxo cations
Have a higher tendency to form complex
Show regular decrease in atomic radii due to actinoid contraction