topic 2 - states of matter and mixtures
1/45
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
describe the arrangement of particles in solid matter
tightly packed
regular pattern
describe the movement of particles in solid matter
vibrate around a fixed position
describe the relative energy of particles in solid matter
low energy
describe the density of particles in solid matter
high density
describe the arrangement of particles in liquid matter
randomly arranged
but all particles are touching
describe the movement of particles in liquid matter
move around each other
describe the relative energy of particles in liquid matter
greater energy than solid particles
describe the density of particles in liquid matter
medium density
describe the arrangement of particles in gas matter
randomly arranged
describe the movement of particles in gas matter
move at a range of speeds
in all directions
describe the energy of particles in gas matter
high energy
describe the density of particles in gas matter
low density
recall the name of the interconversion from solid to liquid
melting
state the physical changes that occur in melting
matter goes from solid to liquid
state the chemical changes that occur in melting
due to temperature increase, particles in solid matter gain kinetic energy
causing the particles to start to move around each other
recall the name of the interconversion from liquid and gas matters
boiling
state the physical changes that occur in boiling
matter changes from liquid to gas state
state the chemical changes that occur in boiling
due to temperature increase, the particles in liquid matter gain kinetic energy and overcome bonds of attraction between particles
causing the particles to move freely at a range of speeds in random directions
recall the name of the interconversion from gas to liquid matters
condensation
state the physical changes that occur in condensation
matter changes from a gas to a liquid state
state the chemical changes that occur in condensation
due to temperature decrease, the gas particles lose kinetic energy
causing the particles to slow and move around each other
recall the name of the interconversion from liquid to solid matter
freezing
state the physical changes that occur in freezing
matter changes from liquid to solid state
state the chemical changes that occur in freezing
due to temperature decrease, the liquid particles lose kinetic energy
causing the particles to slow and start vibrating around a fixed point
recall the name of the interconversion from solid to gas
sublimation
state the physical changes that occur in sublimation
matter changes from a solid to gas state
state the chemical changes that occur in sublimation
due to temperature increase, the solid particles gain kinetic energy and overcoming bonds of attraction between particles
causing the particles to move freely at a range of speeds in random directions
explain the difference between the use of ‘pure’ in chemistry and everyday use
in everyday language, pure means when something is natural or clean
in chemistry, pure means a substance that consists of a single element or compound
which contains no other substances
state the definition of pure in chemistry
pure means a substance that consists of a single element or compound
which contains no other substances
state the differences in chemistry between a pure substance and a mixture
a pure substance contains ONE element or compound CHEMICALLY BONDED together
a mixture contains TWO OR MORE elements or compounds that are PHYSICALLY MIXED together
state how to interpret melting point data to recognise a pure substance
pure substances have sharp melting points
state how to interpret melting point data to recognise a mixture
mixtures have melting range of temperatures
explain the type of mixtures that can be separated by simple distillation
separates a liquid from a soluble solid in a solution
or a pure liquid from a mixture of liquids
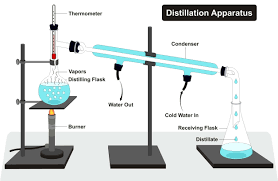
state the method of simple distillation
heat the solution in a distilling flask, using a bunsen burner
as the pure liquid evaporates, the vapour is captured and passes through the liebig condenser
the vapour cools and condenses, turning into a pure liquid that is collected in a beaker
explain the types of mixtures that can be separated by fractional distillation
separates two or more liquids which are miscible
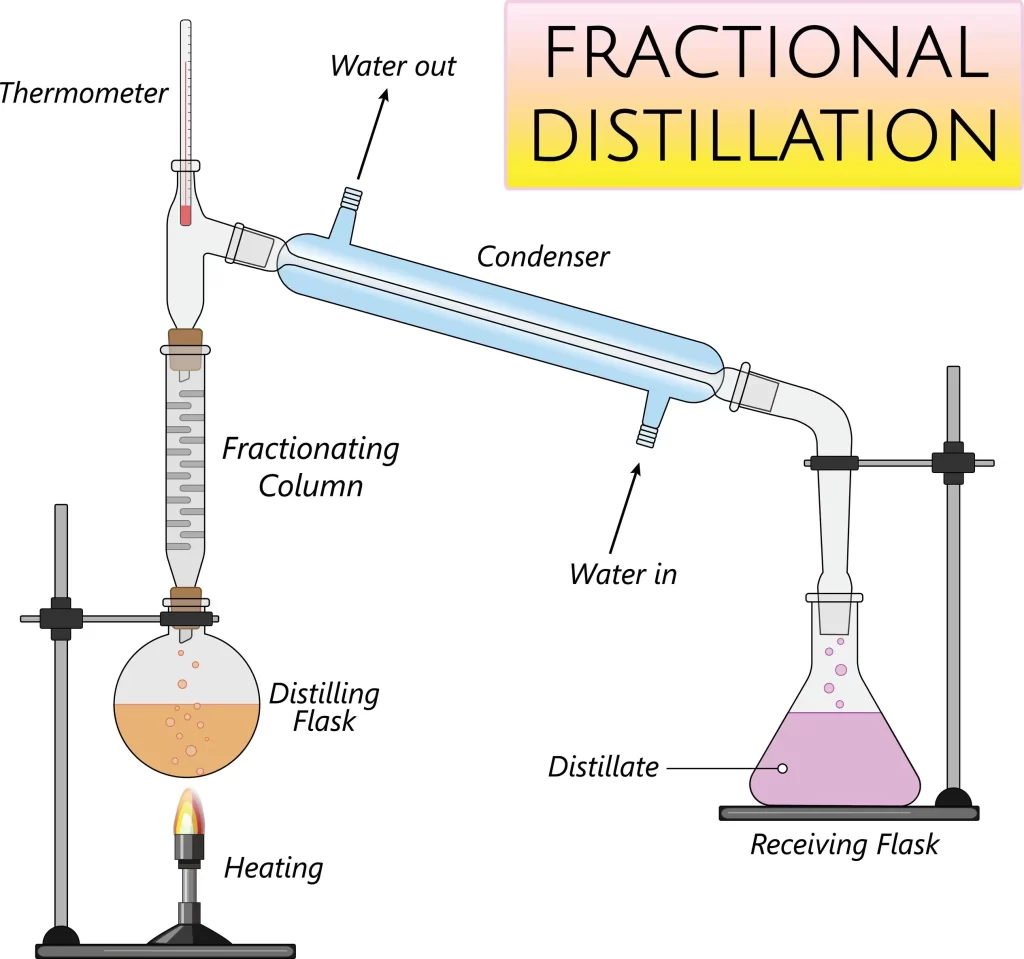
state the method of fractional distillation
set up the apparatus as pictured
heat the solution in a distilling flask, using an electric heater
the solution is heated to the temperature of the substance with the lowest boiling point
the substance with the lowest boiling point evaporates and its vapour is collected in the liebig condenser
as the vapour passes through the condenser, it cools and condenses to form a pure liquid
this liquid is collected in a beaker
repeat the experiment as many times as needed for different types of liquids by changing the temperature on the electric heater
explain the types of mixtures that can be separated by filtration
separates an insoluble solid from a liquid
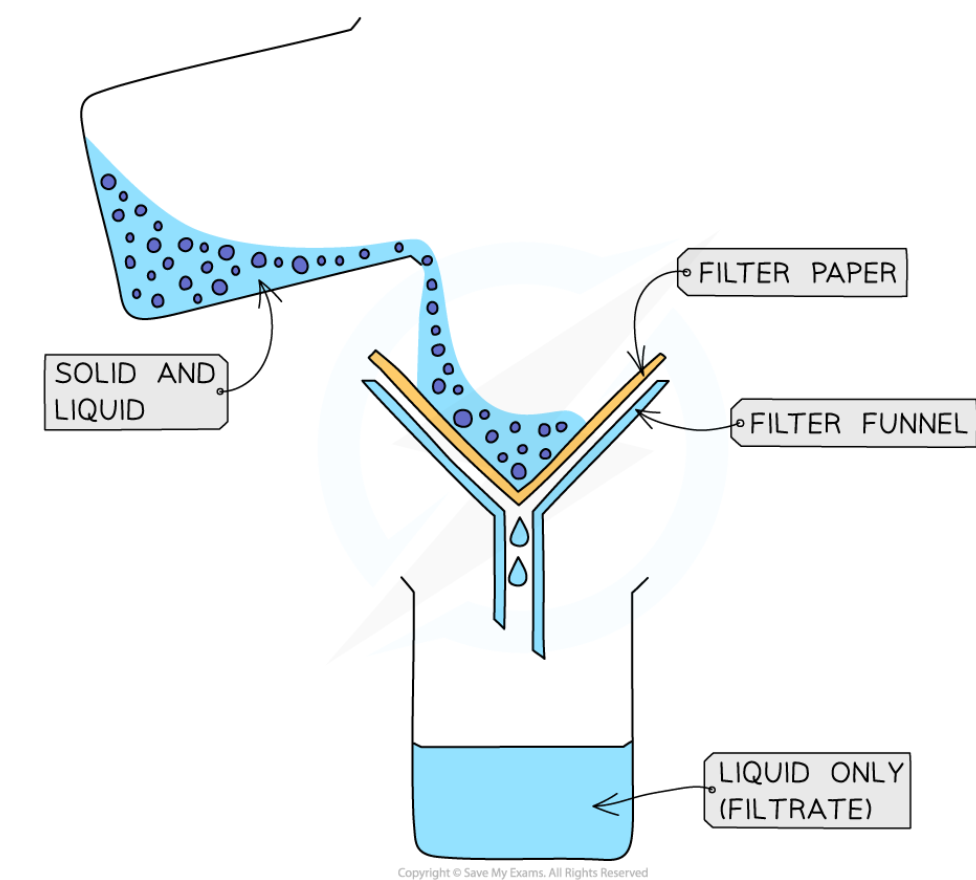
state the method of filtration
place a filter paper in a funnel above a beaker
add the mixture of the insoluble solid and liquid to the filter funnel
the filter will only allow the small liquid particles through as a filtrate
solid particles are too large to pass through the filter paper so remain in the filter funnel as residue
explain the types of mixtures that can be separated by crystallisation
separates a soluble solid from a solution when the solid is more soluble in hot than cold solvents
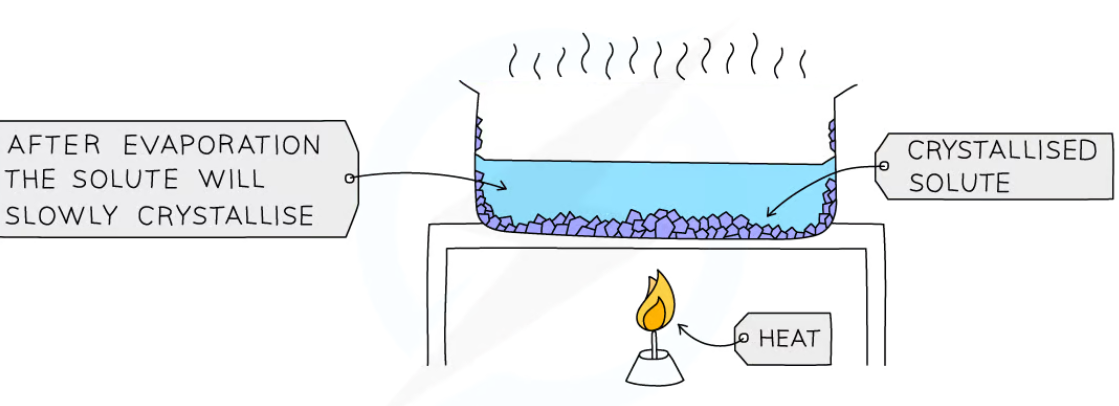
state the method of crystallisation
heat the solution in an evaporating basin until the solvent has evaporated, leaving a saturated solution behind
cool the saturated solution slowly
as the solution cools, crystals will start to form
filter the solution to separate the crystals from the solvent
wash the crystals with distilled water to remove impurities
dry with a paper towel
explain the types of mixtures that can be separated by paper chromatography
separates substances with different solubilities within a solution
state the method of paper chromatography
draw a pencil line horizontally-across a piece of chromatography paper
place a small dot of each substance onto the pencil line equal distances apart
lower the paper into the solvent so that the pencil line sits above the level of the solvent
the solvent travels up the paper by capillary action
the substances with higher solubilities will travel further up the paper than substances with lower solubilities
describe what paper chromatography is
separation of mixtures of soluble substances
by running a solvent (mobile phase)
through the mixture on the paper (paper is stationary phase)
causing the substances to move at different rates over the paper
state how to interpret a paper chromatogram to distinguish between pure and impure substances
pure substances will only produce one spot on the chromatography paper
as the paper shows each different component of a mixture as a spot
impure substances will produce more than one spot on the chromatography paper
state how to calculate Rf values
Rf = distance travelled by substance / distance travelled by solvent
explain how to investigate the composition of inks (method)
use simple distillation to separate the dyes and solvents in the ink
use a pencil to draw a horizontal line across a piece of chromatography paper
add a dot of each known solvent on the pencil line using a different capillary tubes
use another capillary tube to add a dot of the unknown solvent from the ink on the pencil line
lower the chromatography paper into the water until the pencil line is above the level of the solvent
allow the solvents to travel undisturbed up the chromatogram paper
measure the distance travelled by the water and measure the distance travelled by each solvent
calculate the Rf value of each solvent
compare the Rf value of the unknown solvent to the other solvents to discover what the unknown solvent is