Chemistry exam paper quesitons
1/13
Earn XP
Description and Tags
test with whiteboard
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
Write the equation for the reaction of strontium with water
Sr + 2H2O → Sr(OH)2 + H2
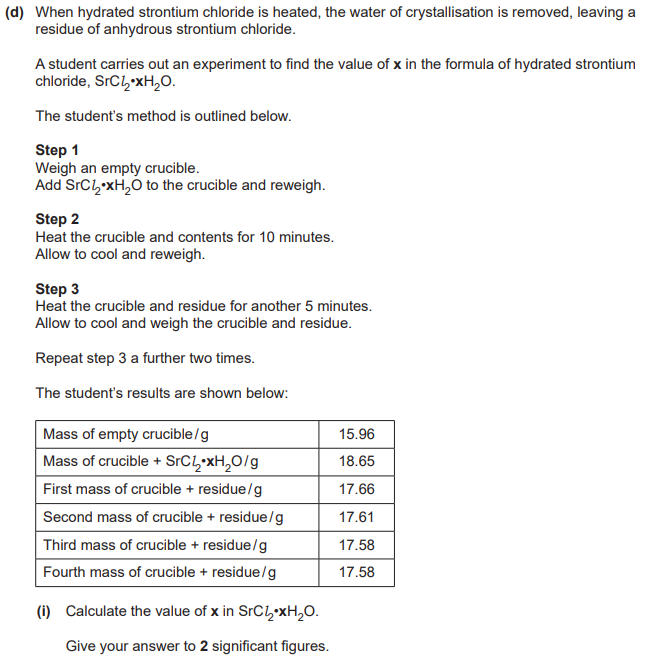
x = 5.8

Level 3 (5-6 marks)
calculates correct mass of hydrous magnesium nitrate/anhydrous magnesium nitrate AND
Explains preparation steps, with most fine detail

2Mg(NO3)2 → 2MgO + 4NO2 +O2
Using oxidation numbers, explain why this is a redox reaction
Both oxidation and reduction takes place
Oxygen is oxidised because it goes from -2 in Mg(NO3)2 to 0 in O2
Nitrogen is reduced because it goes from +5 in Mg(NO3)2 to +4 in NO2
1-iodopentane can be hydrolysed by water using aqueous silver nitrate, with ethanol as a solvent. A student uses this method to compare the rates of hydrolysis of 1-iodopentane and 1-bromopentane.
1-iodopentane reacted faster than 1-bromopentane. Explain why
C-I bond is weaker than C-Br bond/C-I bond has lower bond enthalpy than C-Br bond
carbon-halogen bond breaks (more easily)
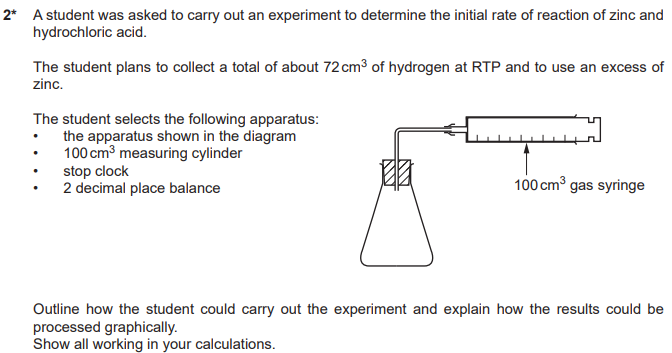
A student reacts Zinc with hydrochloric acid. The rate of reaction decreases over time.
Explain why, in terms of collision theory
acid concentration decreases
fewer collisions per second/less frequent collisions
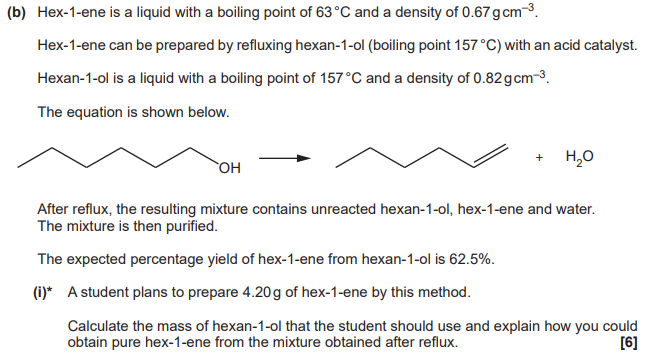
Level 3 (5-6 marks)
Calculates correct mass of hexan-1-ol AND
Explains purification steps, with most fine detail
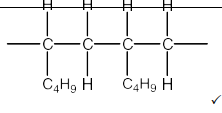
Hex-1-ene can be polymerised to form poly(hex-1-ene).
Draw a section of poly(hex-1-ene) containing two repeat units
Calculate the number of barium ions in 1.50g of barium oxide.
Give your answer in standard form and to three significant figures
5.89 × 1021
Barium nitride is formed when barium is heated with nitrogen.
Write the electron configuration of a nitride ion.
1s22s22p6
Barium nitride reacts with water to form an alkaline solution and an alkali gas.
Write the equation for this reaction, including state symbols
Ba3N2(s) + 6H2O(l) → 3Ba(OH)2(aq) + 2NH3(g)
Level 3 (5-6 marks)
All three scientific points are covered in detail and explained thoroughly
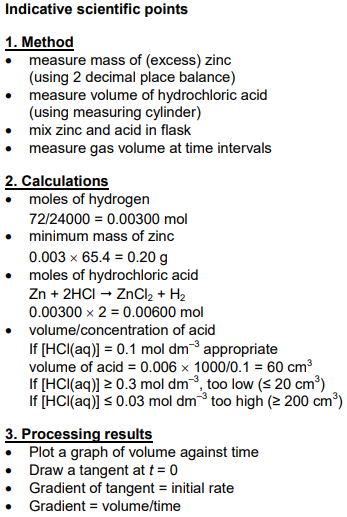
Describe the oxidation reactions of butan-1-ol forming an aldehyde and a carboxylic acid.
Explain, using a diagram, how the aldehyde can be produced in the laboratory by controlling the reaction conditions [6]
Level 3 (5-6 marks)
A comprehensive explanation with all three specific points covered thoroughly
A student carries out a titration to determine the mass of citric acid in one lime. The student follows the method:
Squeeze the juice out of two limes
transfer the juice onto a 250.0cm3 volumetric flask and make up to the mark with distilled water
Pipette 25.0cm3 of the diluted lime juice into a conical flask and add a few drops of indicator
Titrate solution with 0.8 mol dm-3 NaOH(aq)
They record and calculate a mean titre of 27.35cm3
Citric acid is neutralised by NaOH as shown by the equation below:
C6H8O7 + 3NaOH → Na3C6H5O7 + 3H2O
Calculate the mass, in g, of citric acid in one lime, assume that citric acid (Mr = 192.0) is the only acid in lime juice
Suggest how the student could modify the method to use an NaOH concentration of 0.2 mol dm-3 instead of 0.8 mol dm-3 . The student should aim to have the same titre as in the original method. Justify your answer
7g
use half a lime OR make up lime juice solution to 1 dm3 volumetric flask
(Justification) 4 times less citric acid OR NaOH is 4 times more dilute (giving same titre) OR 1:4 ratio for NaOH concentration