CHEM Elements, compounds and mixtures
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
Characteristics of elements
It is a pure substance
It is made up of only one kind of atoms
It can’t be separated into simpler substances
It is divided into metals, non metals, matalloids, and noble gases
Characteristics of compounds
It is a pure substance
It is formed by the combination of two or more different elements
It can be broken down into its elements by chemical methods only
Characteristics of mixtures
It is an impure substance
It is formed by the combination of two or more elements, compounds or both
It retains the properties of its constituent elements or compound
Element
It is a pure substance made up of only one kind of atoms
Structure of an atom
Atom is the smallest partical of an element which can take part in a chemical reaction. It is divided into
Nucleus-
Protons - positively charged particals.
Neutrons - particals having no charge.
Orbits/Shells/energy levels
Electrons - negatively charged particals.
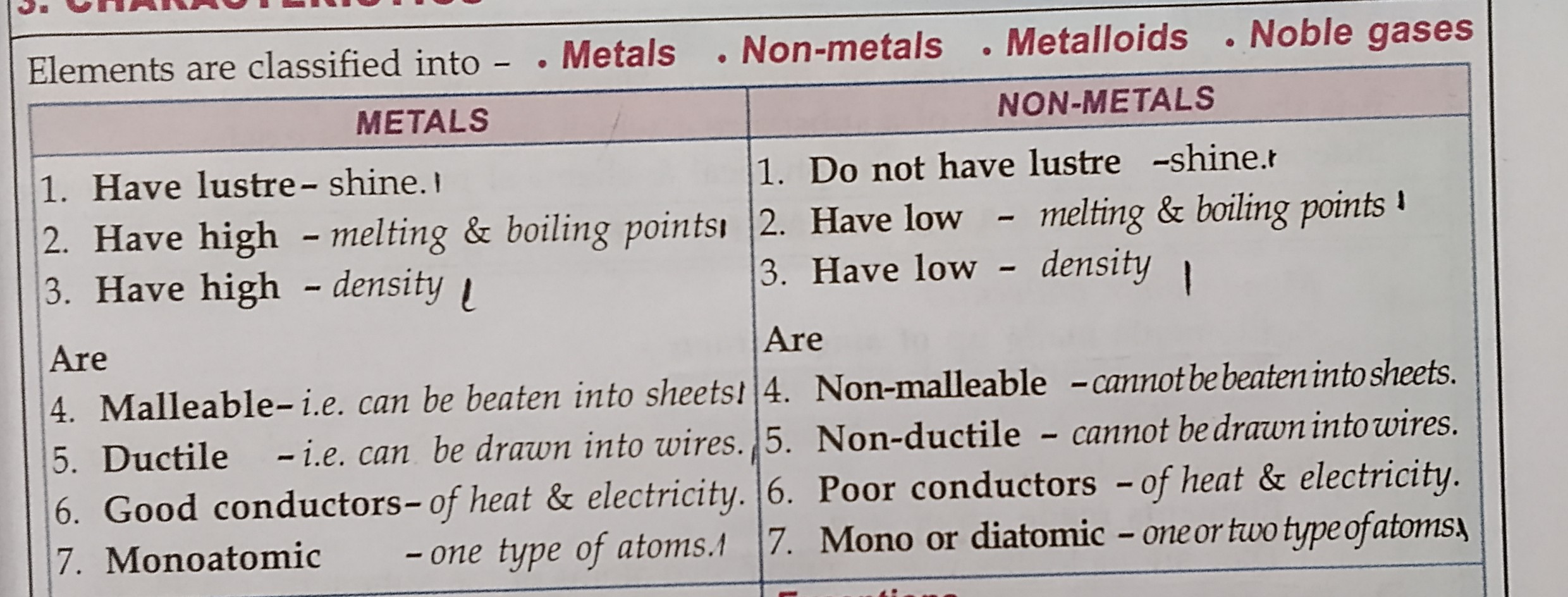
Difference between metals non metals
LEARN
Differences between metalloids and noble gases
LEARN

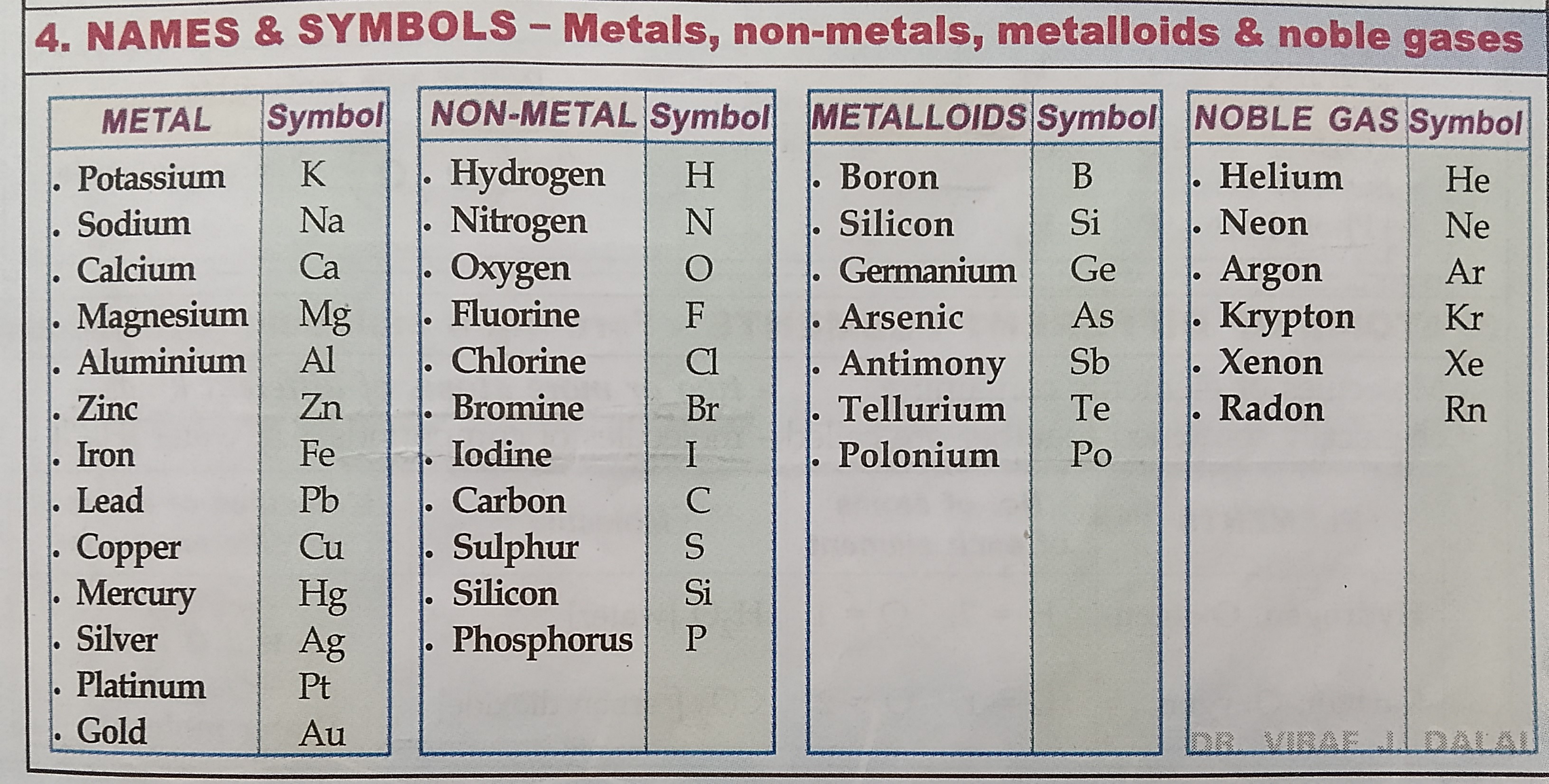
Names and symbols of elements
LEARN
Molecules
Atoms of the same or different elements combine to form a molecule
Monoatomic molecules
Elements made up of single atoms
Diatomic elements
Elements made up two of atoms
Poly atomic molecules
Elements made up of more that two atoms
Atomicity
The number of atoms of an element that joint together to form a molecule of an element
Molecules of compounds
When two or more atoms of different kinds chemically combine together are called molecules of compounds.
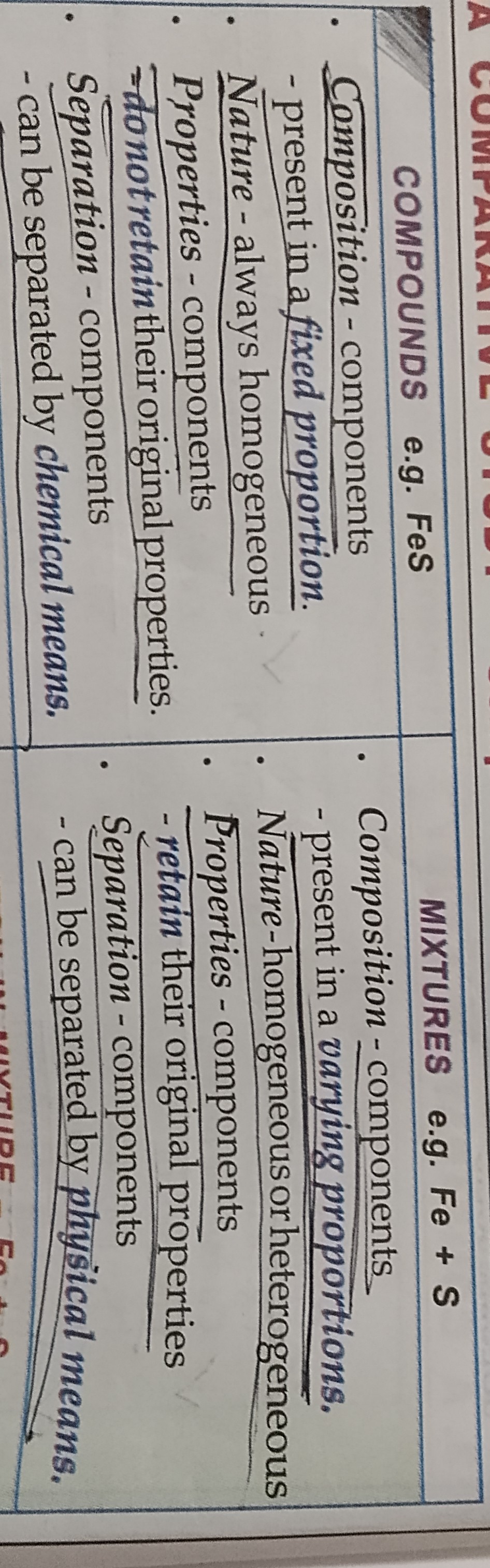
Compound
An pure substance made up of two or more different elements chemically combined together in a fixed proportion
Properties of a compound
Components in a compound arr in a fixed proportion
Particals in a compound are of one kind
Components of a compound have a definite set of properties
Elements in a compound do not retain their properties
Components of a compound can be separated by chemical methods only
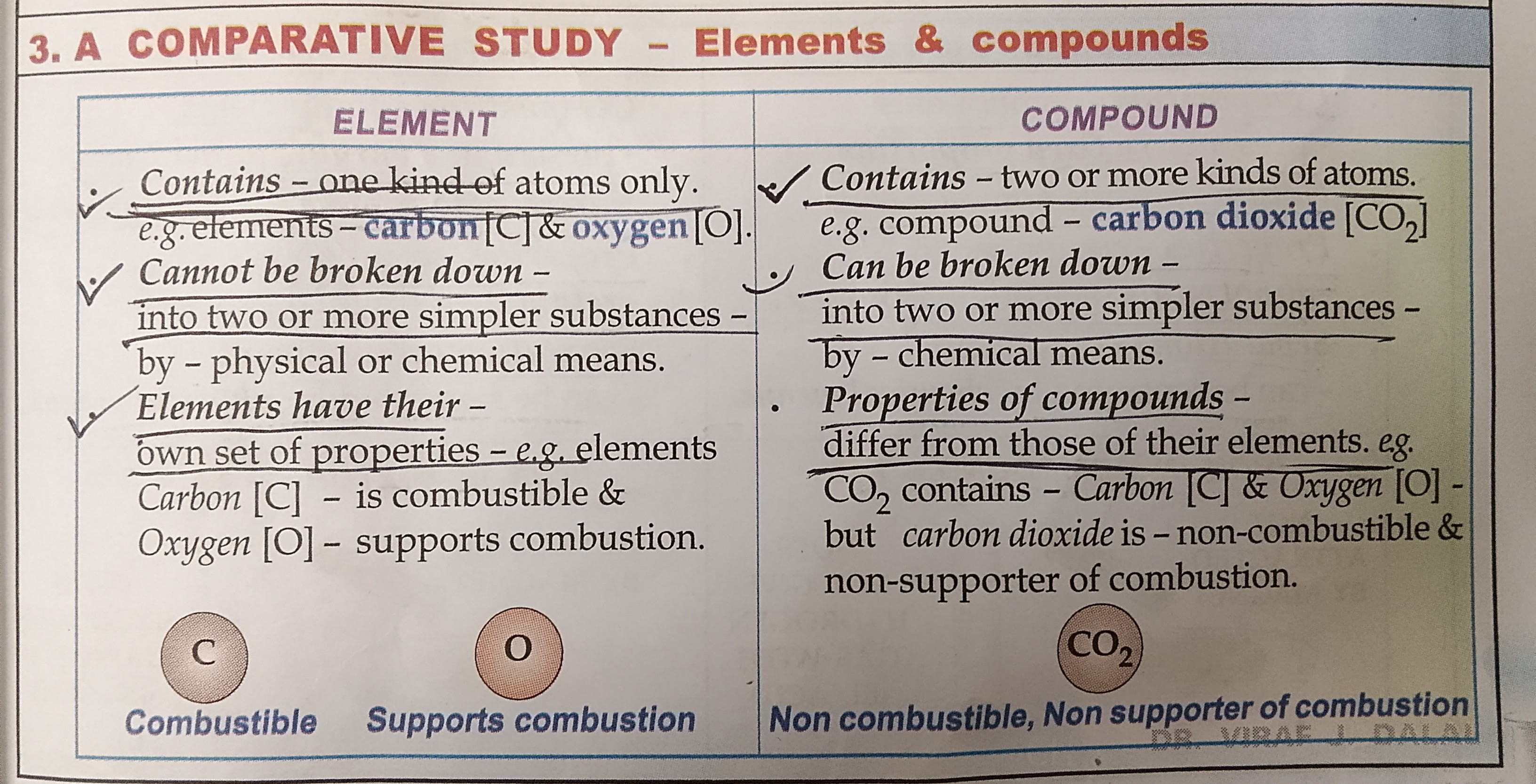
Differenciate between elements and compound
LEARN
Mixtures
It is an impute substance made up of two or more elements or compounss or both combined mechanically in any proportion. A mixture retains the propertirs of its constituents.
Homogeneous mixtures
A mixture in which the constituents are uniformly mixed throughout.
Heterogeneous mixtures
Mixtures inwhich the constitients are not uniformly mixed throughout.
Differences between homogeneous mixtures and heterogeneous mixtures
LEARN

Differences between compounds and mixtures
LEARN
Name the 4 ways to separate solid-solid mixtures
Separation methods are-
Sublimation
Magnetic separation
Solvent extraction
Fractional crystillisation
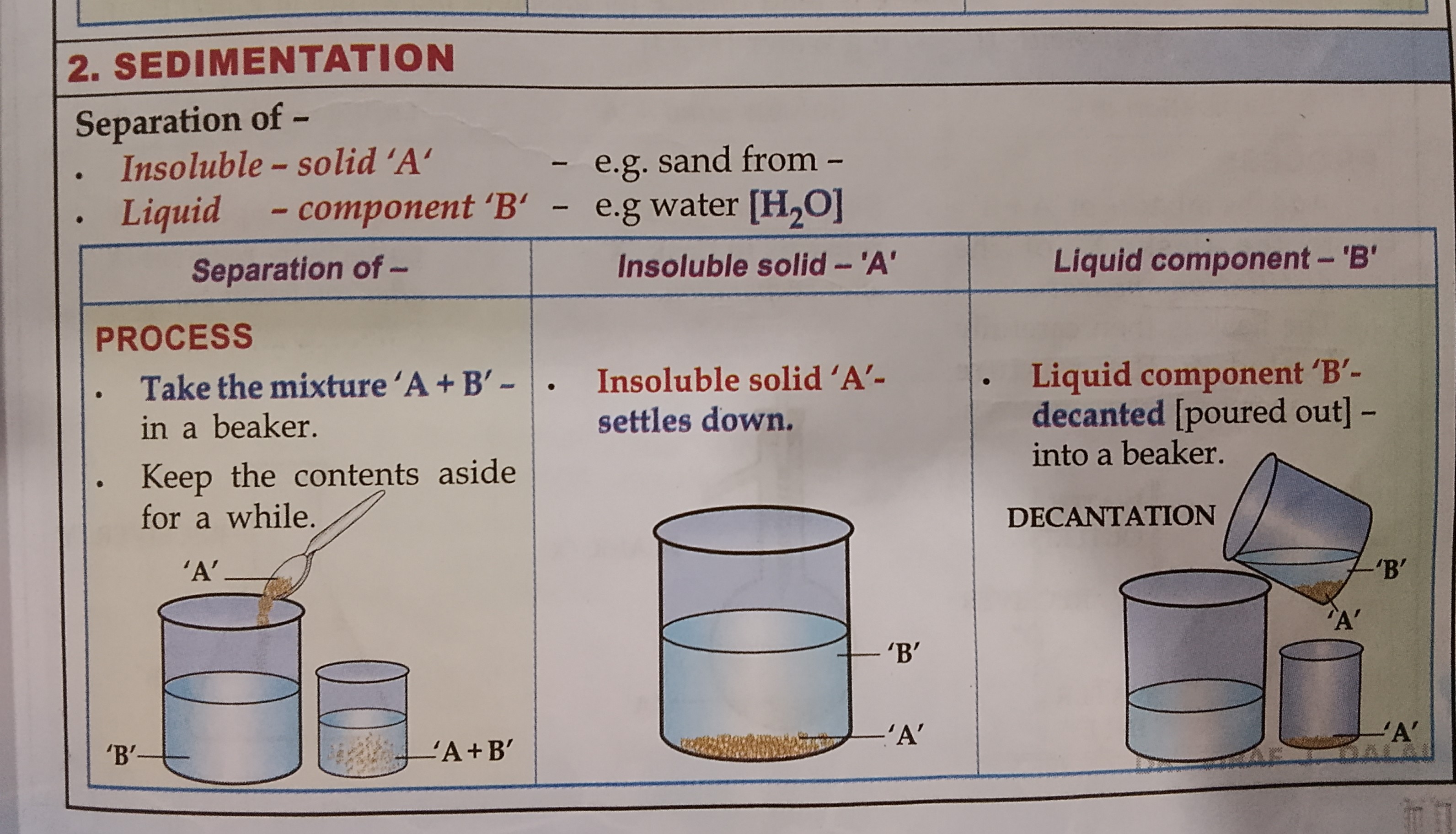
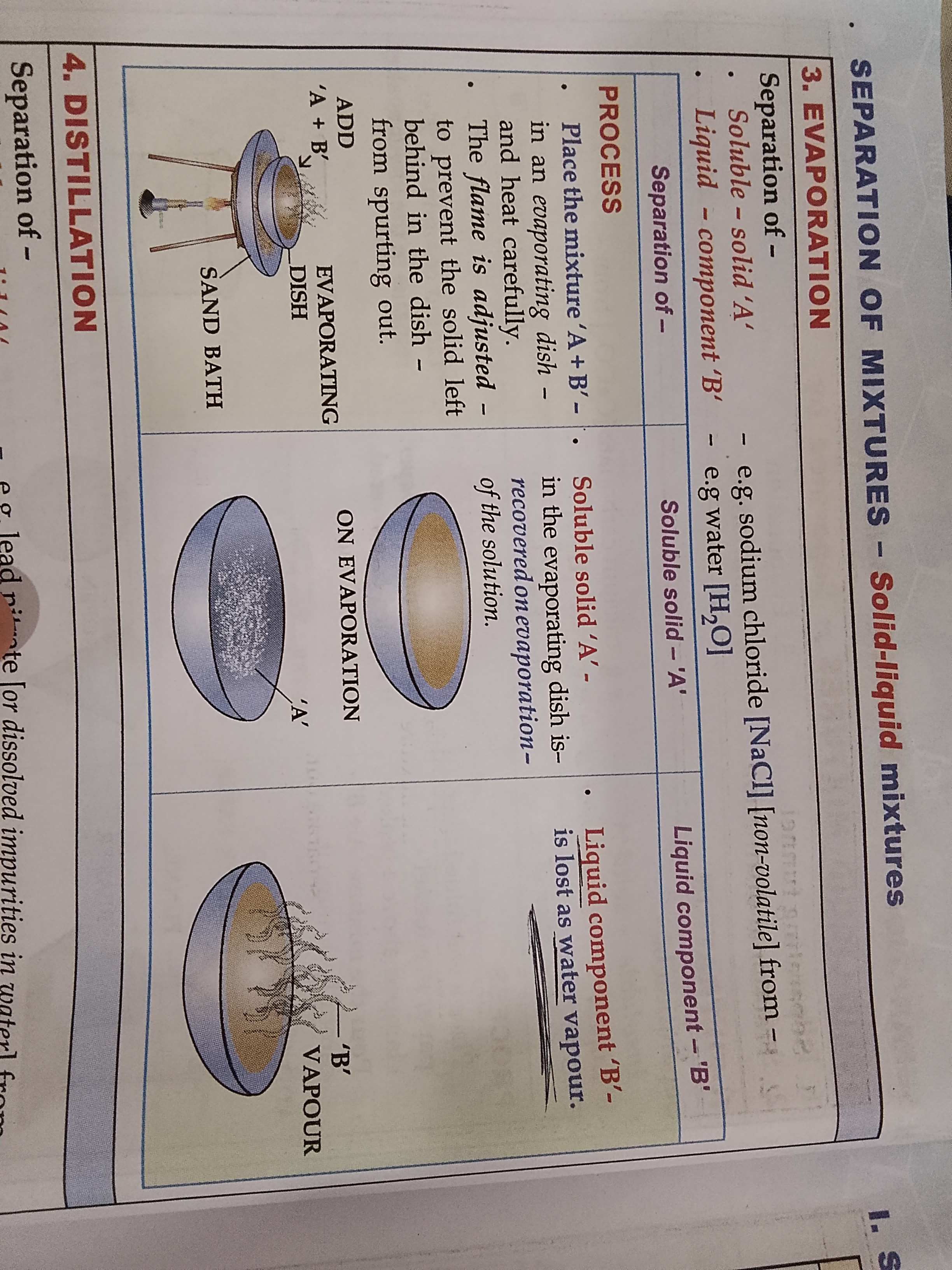
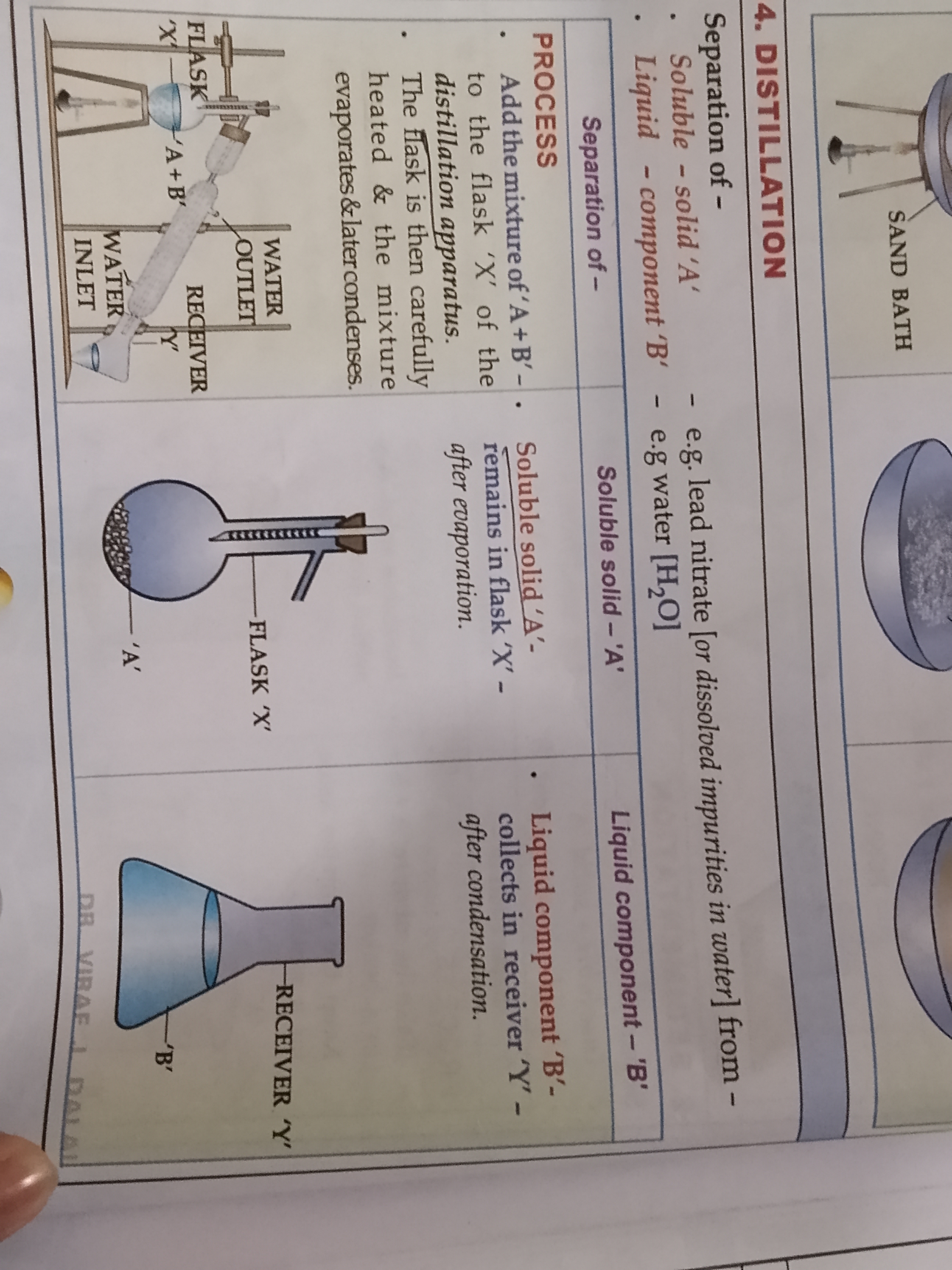
Name the 4 ways to separate solid-liquid mixtures
The separating methods are
Filteration
Sedimentation
Evapouration
Distillation
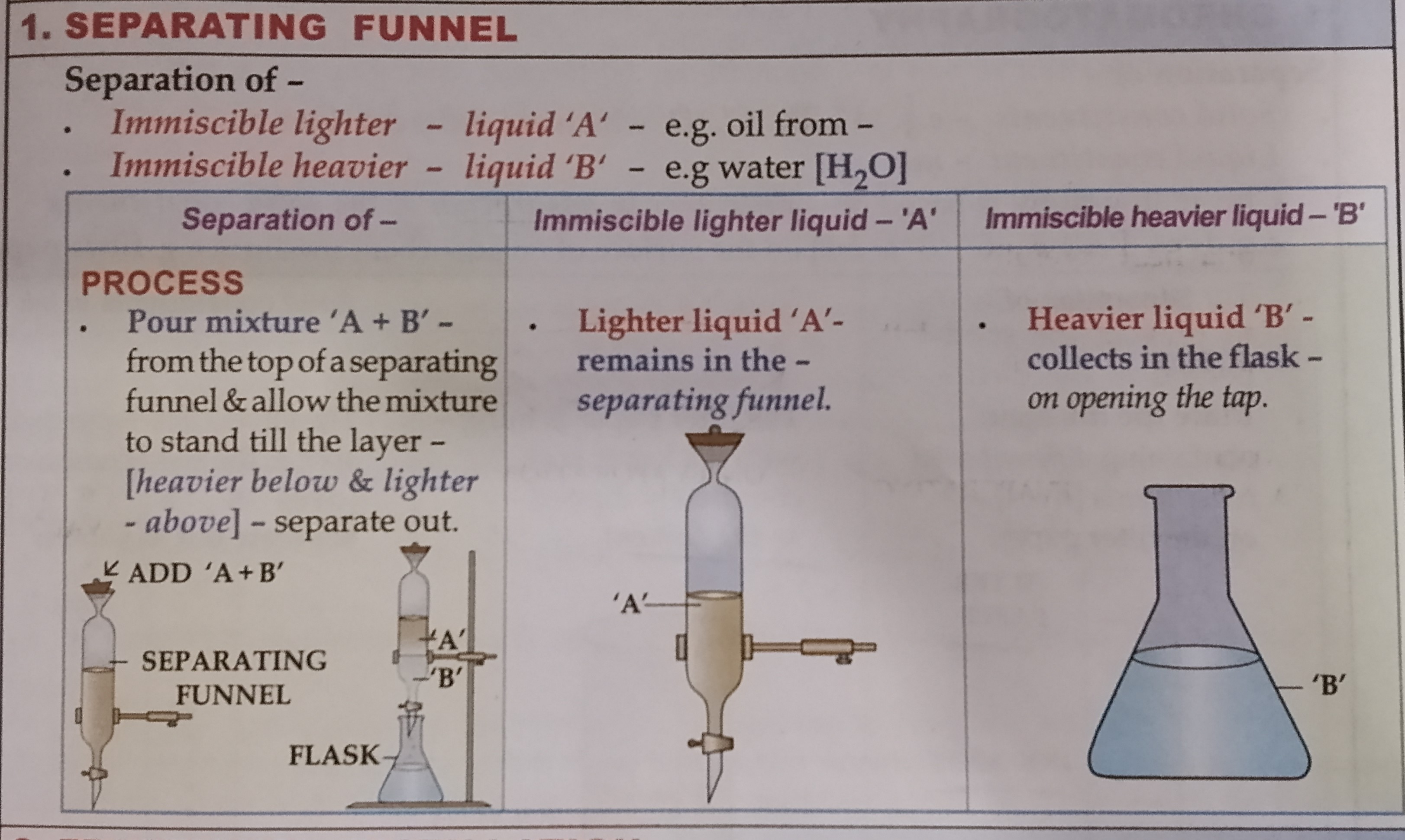
Name the 2 methods of separating liquid-liquid methods
The 2 methods of separation are
Separation funnel
Fractional disstillation
Name the method to separate liquid gas mixtures
Boiling the liquid gas mixtures
Name the 2 methods of separating gas-gas mixtures
Diffision
Solubility in solvent
Name 2 methods used to separate complex mixtures
Centrifugation
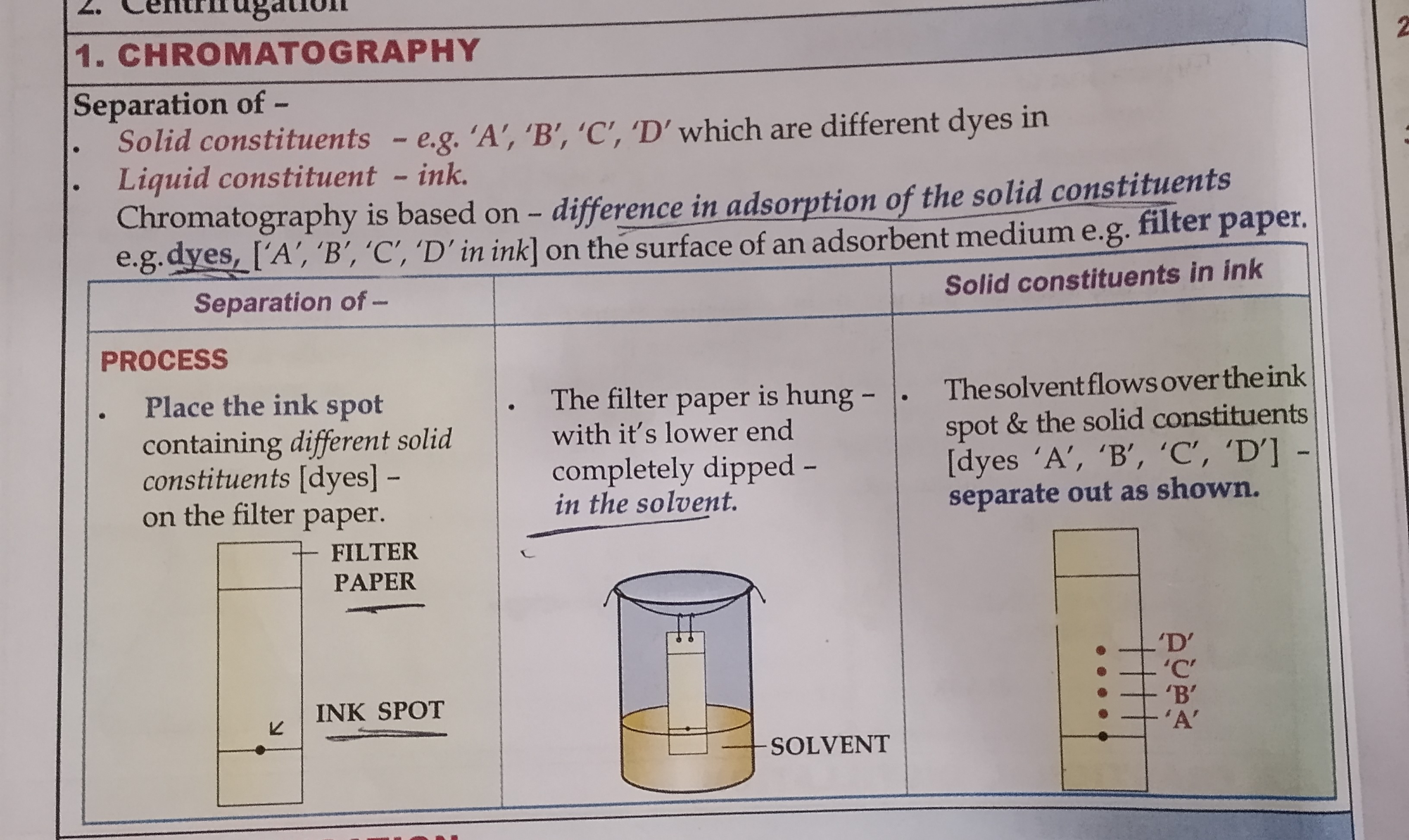
Cromatography
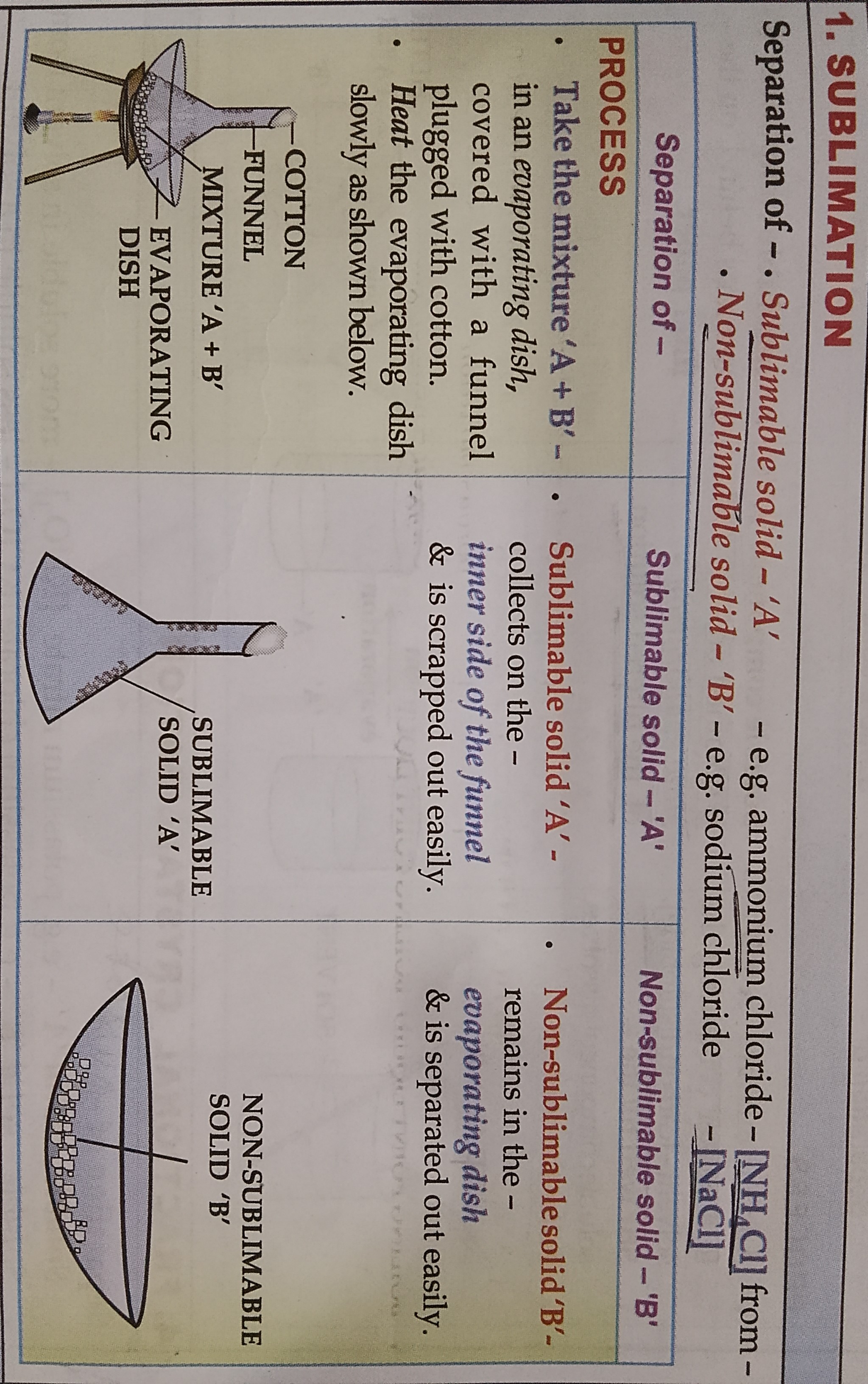
SUBLIMATION
LEARN
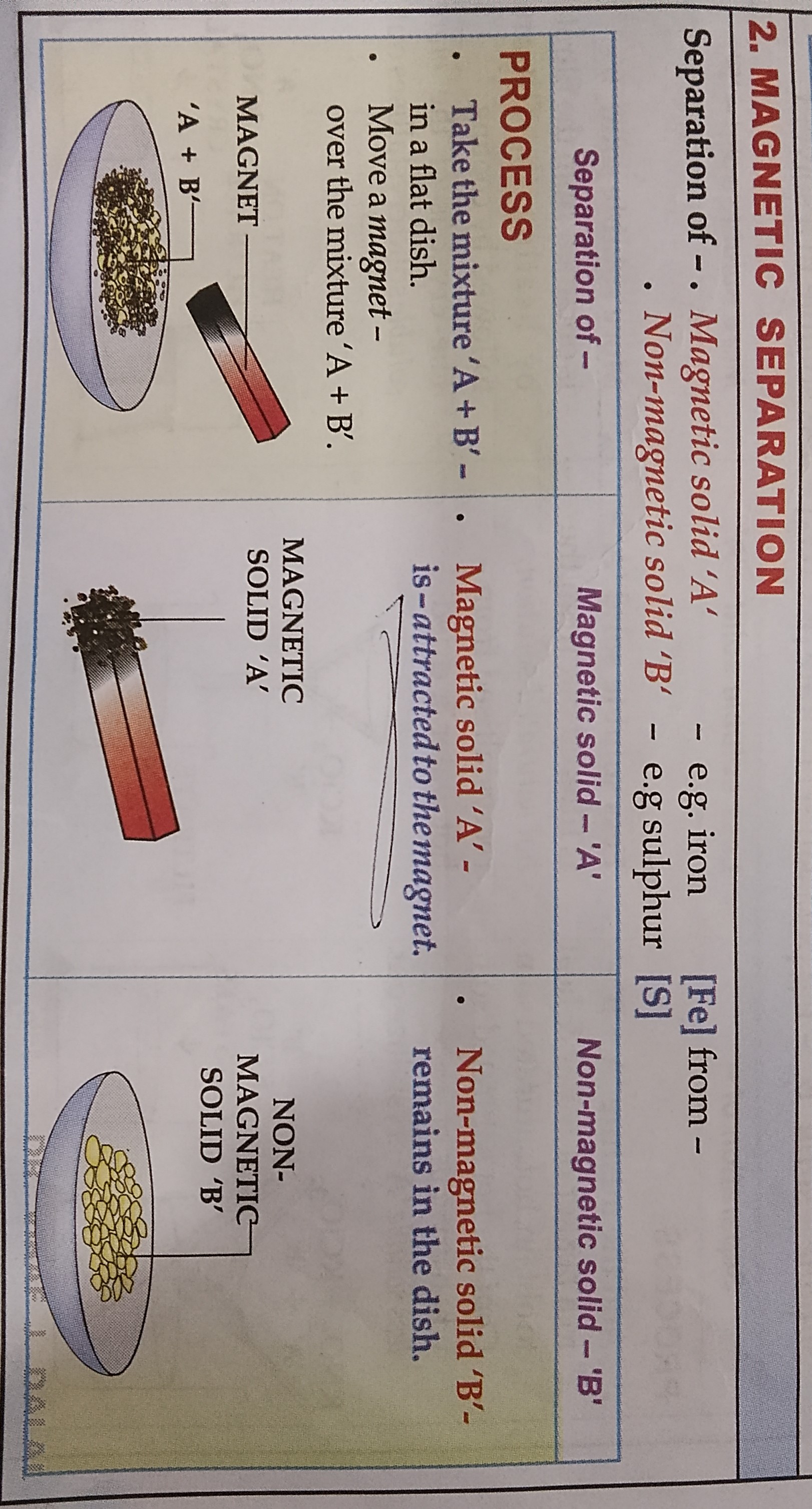
MAGNETIC SEPARATION
LEARN
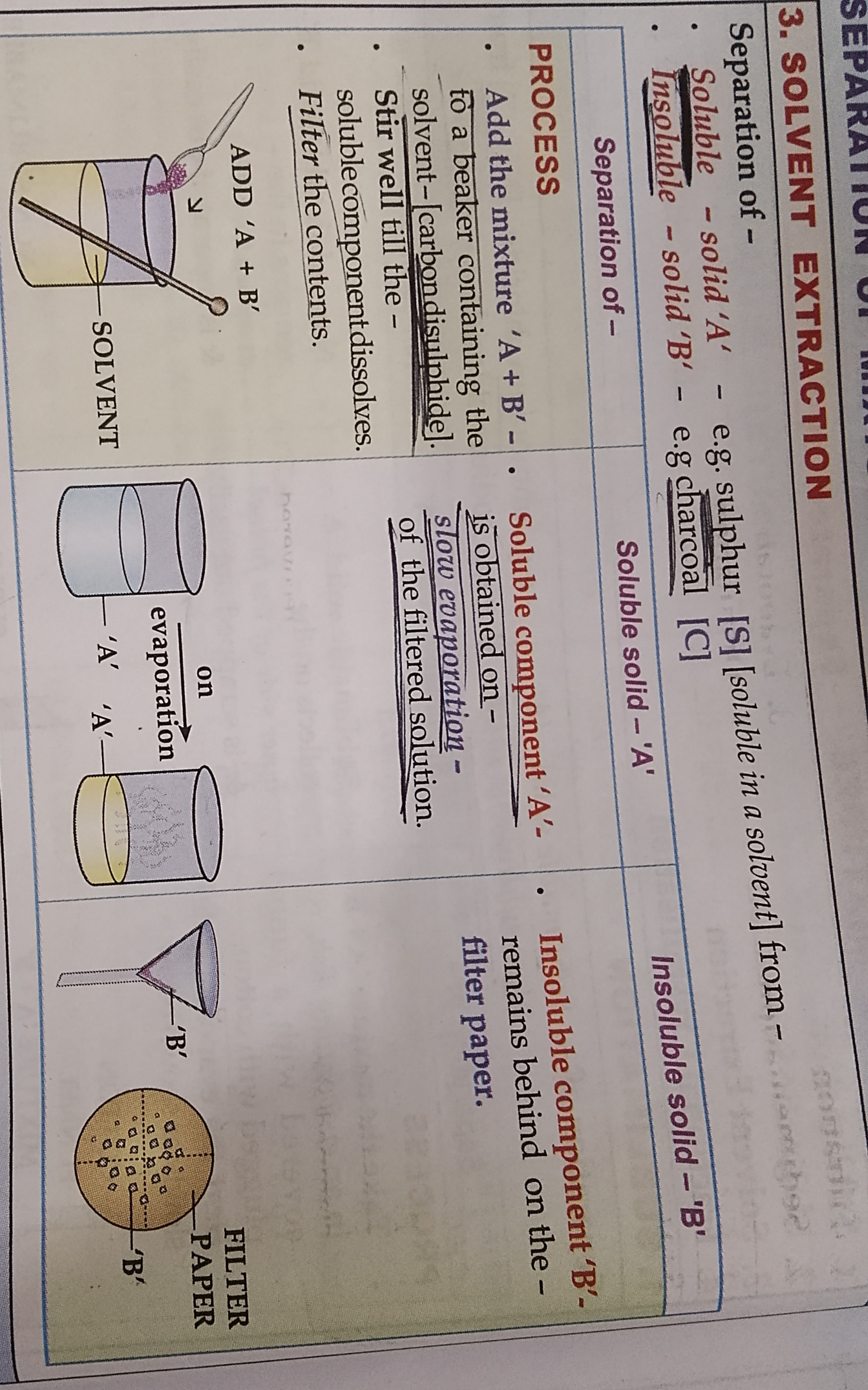
SOLVENT EXTRACTION
LEARN
FRACTIONAL CRYSTILLISATION
LEARN

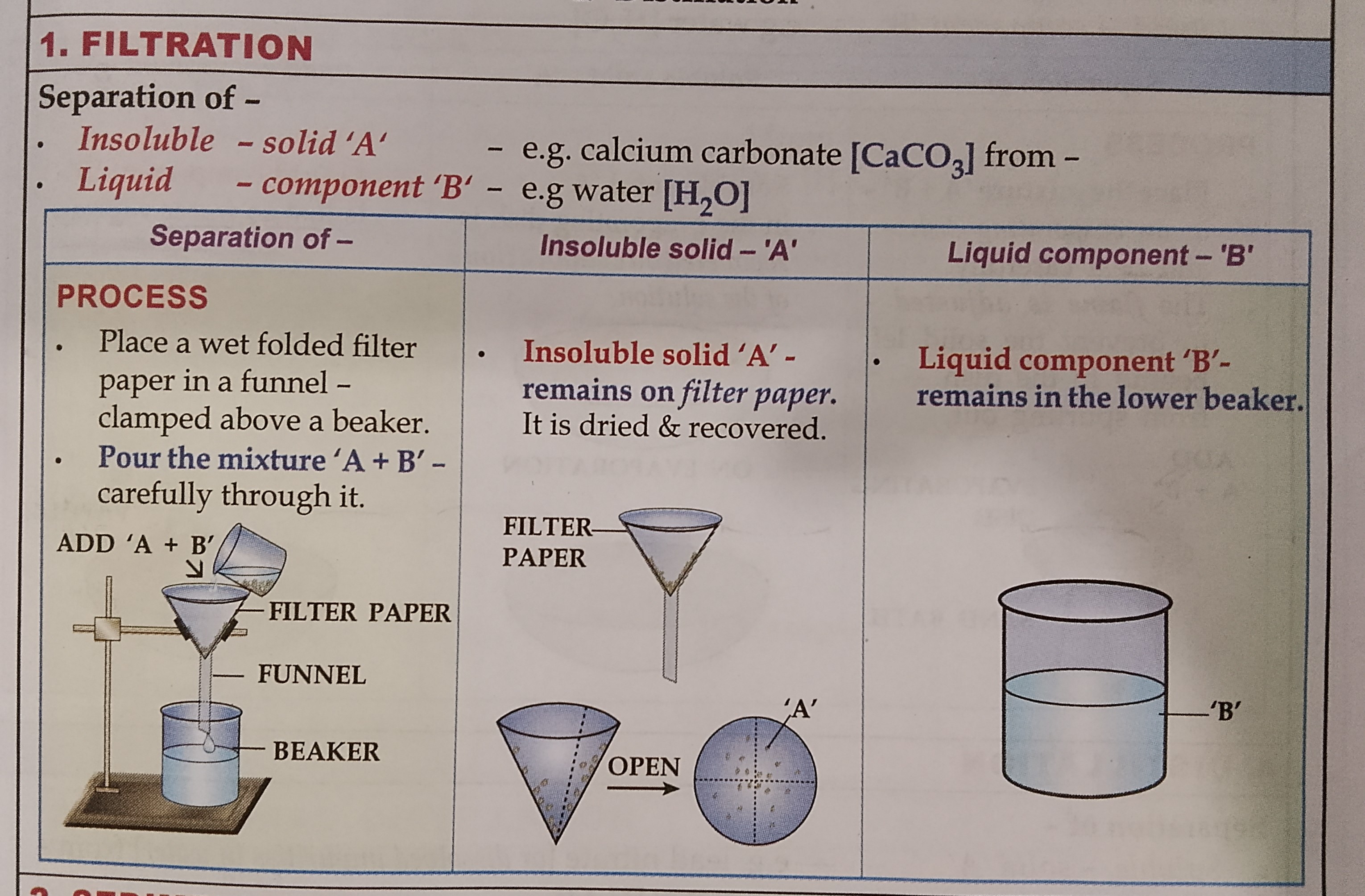
FILTERATION
Learn
SEPARATING FUNNEL
Learn
EVAPORATION
Learn
DISTILLATION
Learn
SEPARATING FUNNEL
Learn
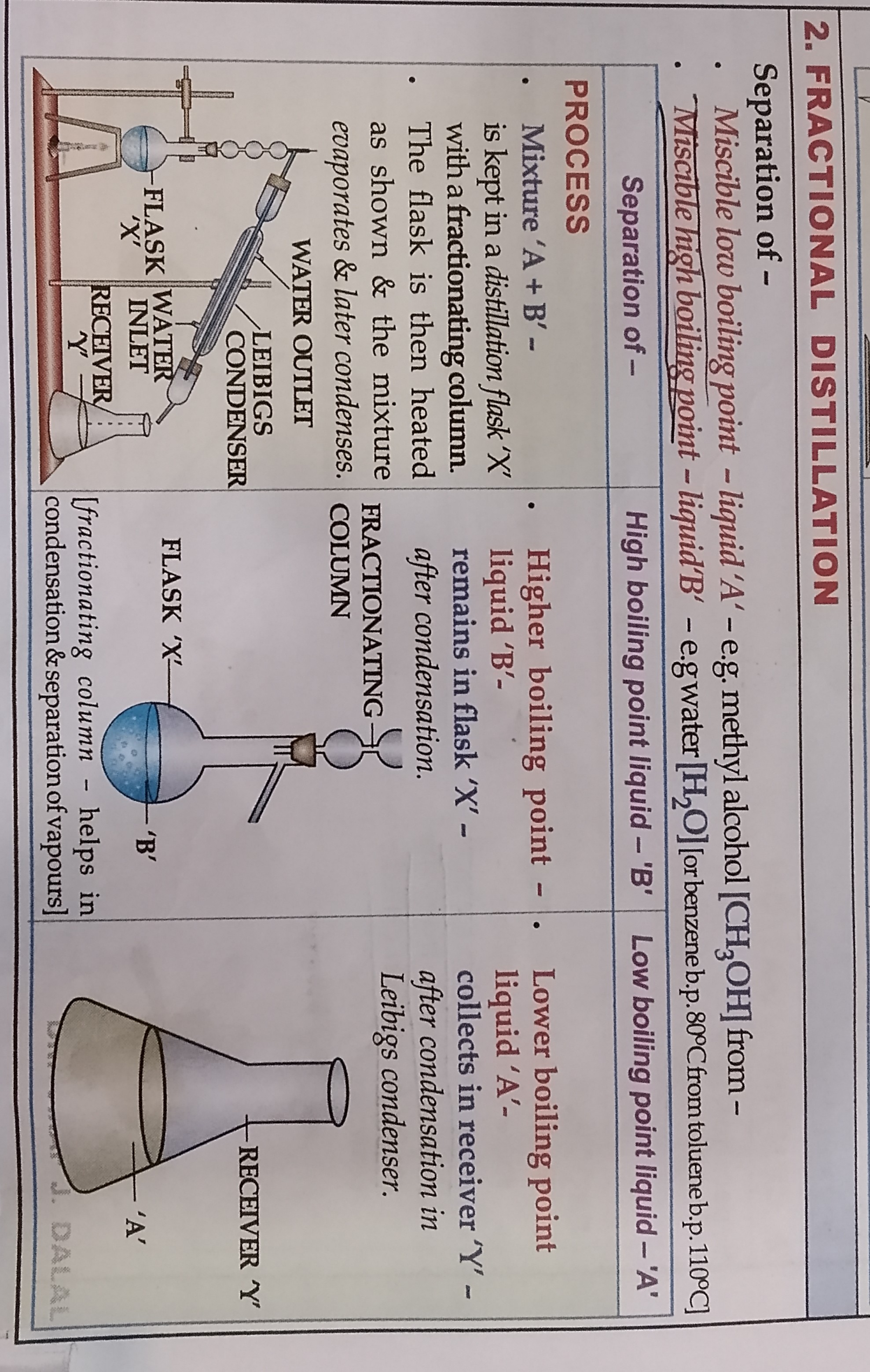
FRACTIONAL DISTILLATION
CROMATOGRAPHY
LEARN
CENTRIFUGATION
LEARN
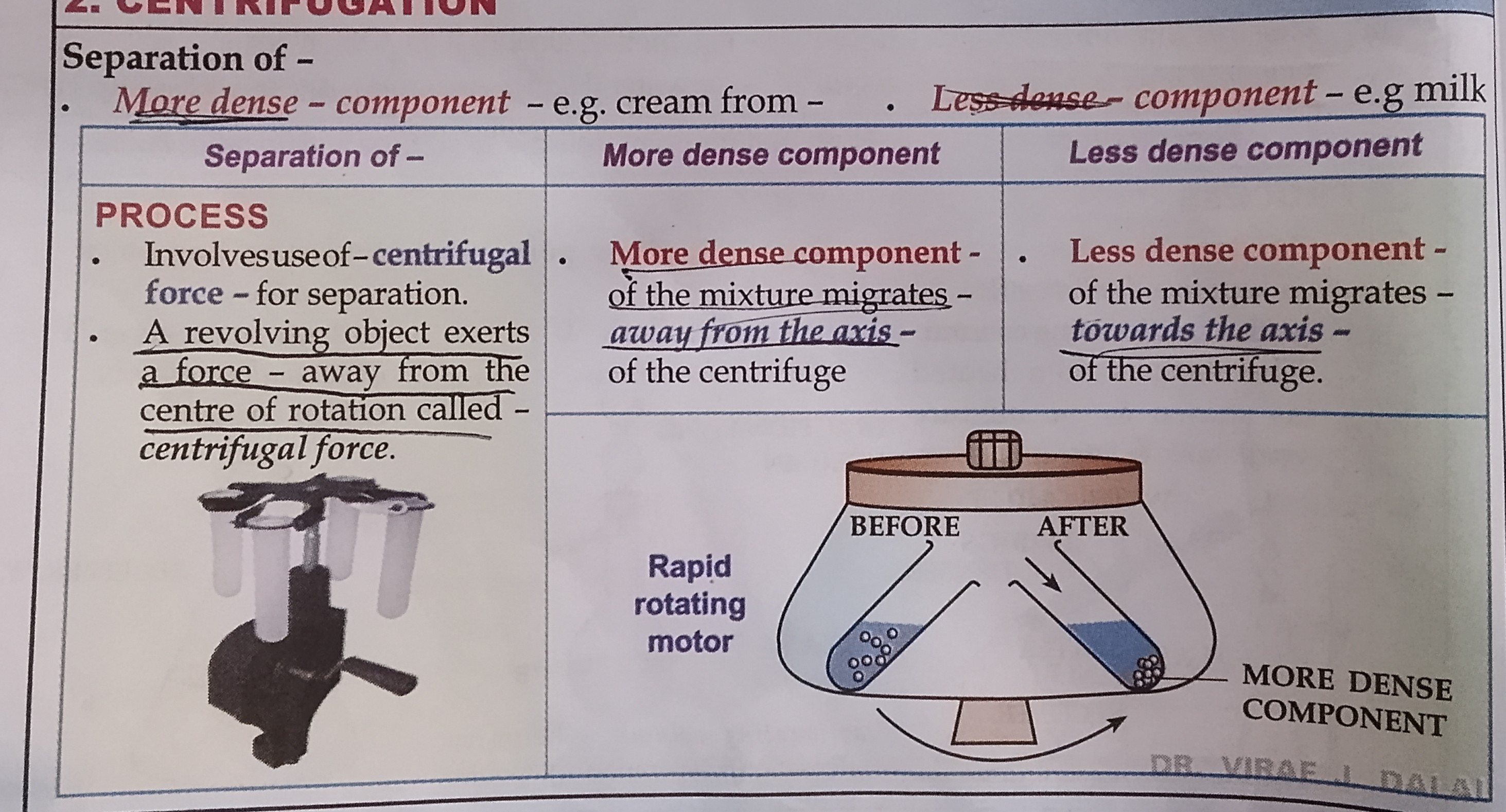
Centrifugal force
A revolving body exerts a force away from the center of rotation called centrifugal force