chapter 2
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
41 Terms
2.1 Characterizing Subatomic Particles Name the subatomic particles that make up all atoms, and the charge and relative mass associated with each.
-The subatomic particles found in all atoms are the proton, electron, and neutron.
-Protons have a positive charge.
-Electrons have an opposite negative charge.
-Neutrons have no charge (neutral).
-The proton and neutron have a relative mass of 1 amu each, and the electron is about 1
2.1 Where are the subatomic particles located in an atom?
Protons and neutrons are located in the nucleus (center) of an atom, and the electrons are found in constant motion around the nucleus, in a space called the electron cloud.
2.2 How does the mass of a proton compare to the mass of a neutron?
The mass of a proton is almost the same as the mass of a neutron, but the neutron is slightly heavier than the proton.
2.3 How does the mass of an electron compare to the mass of a proton?
The mass of an electron is about 2000 times smaller than that of a proton.
2.4 What units are used for describing the mass of a proton or neutron?
atomic mass unit (amu)
2.5 How can you determine the following?
the number of protons present in an atom
the number of neutrons present in an atom
the number of electrons present in an atom
The number of protons (or electrons) is the atomic number.
The number of neutrons is the mass number minus the atomic number.
The number of electrons is the same as the number of protons in an atom.
2.6 What can be determined from the following?
the mass number
the atomic number
the mass number minus the atomic number
total number of protons and neutrons. (その atom の nucleus に含まれる protons + neutrons の合計がわかる。)
protons number and electrons number in this atom. (electrons number = protons number)
neutrons number (mass number - atomic number = neutrons)
2.7 Provide the name and atomic symbol of the element that has the following atomic number:
7
16
50
30
27
nitrogen, N
sulfur, S
tin, Sb
zinc, Zn
cobalt, Co
2.8 Provide the name and atomic symbol of the element that has the following atomic number:
79
19
82
33
11
gold, Au
potassium, K
lead, Pb
arsenic, As
sodium, Na
2.9 What is the mass number for an oxygen atom that contains 8 neutrons?
16
oxygen — atomic number is 8, protons and neutrons is 8 → mass number = protons + neutrons, 8+8=16
2.10 What is the mass number for a fluorine atom that contains 10 neutrons?
19
fluorine atom number is 9.
mass number= 9 protons + 10 neurons = 19
2.11 Determine the number of protons, neutrons, and electrons in the following atoms:
an iodine atom that has a mass number of 131
a potassium atom that has a mass number of 39
53 protons, 78 neutrons, 53 electrons
19 protons, 20 neutrons, 19 electrons
2.12 Determine the number of protons, neutrons, and electrons in the following atoms:
a helium atom that has a mass number of 4
a carbon that has a mass number of 14
protons 2, neutrons 2, electron 2 — atomic number 2 = protons 2 = electron 2, neutrons = mass number−protons 4 -2 = 2
protons 6, neutrons 8, electrons 6 —- atomic number 6 = protons 6 = electrons 6, 14 - 6 = 8
2.13 Write the symbolic notation for atoms with
5 protons and 6 neutrons.
35 protons and 46 neutrons.
14 protons and 14 neutrons.
54 protons and 70 neutrons.
mass number = proton + neutron
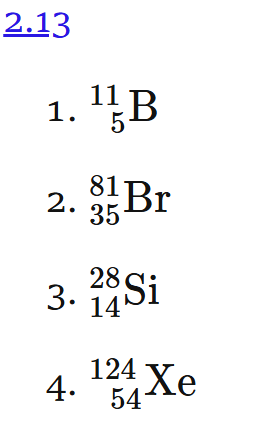
2.14 Write the symbolic notation for atoms
with 3 protons and 4 neutrons.
7 protons and 10 neutrons.
15 protons and 16 neutrons.
46 protons and 60 neutrons.

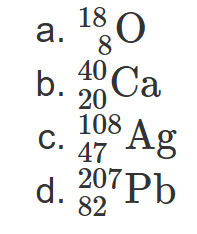
2.15 Determine the number of protons, neutrons, and electrons for each of the following atoms:
8 protons, 10 neutrons, 8 electrons
20 protons, 20 neutrons, 20 electrons
47 protons, 61 neutrons, 47 electrons
82 protons, 125 neutrons, 82 electrons
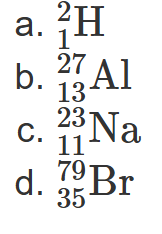
2.16 Determine the number of protons, neutrons, and electrons for each of the following atoms:
1 proton, 1 neutron, 1 electron
13 protons, 14 neurons, 13 electrons
11 protons, 12 neurons, 11 electrons
25 protons, 67 neurons, 25 electrons
What is the relationship between the number of protons and the number of electrons in an atom?
In a neutral atom, the number of electrons is equal to the number of protons.
How would you define isotope?
They have the same atomic number but different mass numbers, so their numbers of neutrons are different.
Where are the protons, neutrons, and electrons located in an atom?
Protons and neutrons are found in the nucleus, while electrons occupy the space around the nucleus
What do all calcium isotopes have in common? How do they differ?
All calcium isotopes have 20 protons. They differ in the number of neutrons, which gives them different mass numbers.
Where is most of the mass of an atom?
Most of the mass of an atom is located in the nucleus, where the protons and neutrons are.
2.4 Isotopes and Atomic Mass Copper has two naturally occurring isotopes:
copper-63 and copper-65. Based on the atomic mass from the periodic table, which of these is more abundant?
The atomic mass of copper (element number 29) is 63.55 amu. Because the average atomic mass is closer to 63 than 65, there must be more copper-63 atoms present in the natural world than copper-65 atoms.
2.17 The mass of the isotope carbon-12 is exactly 12 amu. The atomic mass for carbon (as seen on the periodic table) is 12.01 amu. Explain this difference.
The isotope carbon-12 contains exactly 6 protons and 6 neutrons. The atomic mass as seen on the periodic table is an average mass, taking into consideration the abundance of all the carbon isotopes. Because about 1% of the carbon isotopes are carbon-13, the atomic mass is slightly higher than 12 amu.
2.18 How are atomic mass and mass number similar? How are they different?
Similarities:
→ Both are related to the mass of an atom.
Differences:
→ Mass number = Number of protons + Number of neutrons (integer)
→ Atomic weight = Weighted average of isotopes (decimal)
2.19 There are three naturally occurring isotopes of magnesium: magnesium-24, magnesium-25, and magnesium-26.
How many neutrons are present in each isotope?
Write complete symbolic notation for each isotope.
Based on the average atomic mass given in the periodic table, which isotope of magnesium is the most abundant?
Magnesium-24 has 12 neutrons, magnesium-25 has 13 neutrons, and magnesium-26 has 14 neutrons
24/12Mg,25/12Mg,26/12Mg
3. magnesium-24
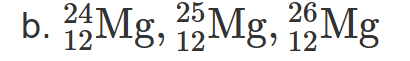
2.20 Some of the naturally occurring isotopes of tin are tin-118, tin-119, tin-120, and tin-124.
How many neutrons are present in each isotope?
Write complete symbolic notation for each isotope.
tin-118 has 68 neutrons, tin-119 has 69 neutrons, tin-120 has 70 neutrons, tin-124 has 74 neutrons.
118/50Sn, 119/50Sn, 120/50Sn, 124/50Sn
2.5 Properties of Radiation Based on its penetrating power into tissue, which form of ionizing radiation is the least dangerous?
An alpha radiation is the least penetrating and does not pass through the skin.
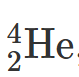
2.21 If the symbol for an alpha particle is 4/2He, explain how an alpha particle differs from a helium atom.
An alpha particle is a helium nucleus. There are no electrons in an alpha particle.
2.22 What is the charge on a beta particle? An alpha particle? A positron?
In some atoms with an unstable nucleus, one of the neutrons may eject a high-energy electron.
2.23 Which form of ionizing radiation has the greatest penetrating power?
neutrons
2.24 Which form of ionizing radiation is most similar to X-ray radiation? How is it different?
Gamma radiation is most similar to X‑ray radiation because both are high‑energy electromagnetic waves with no mass and no charge.
They differ in their origin: X‑rays come from electron transitions, while gamma rays come from changes in the atomic nucleus.
2.25 How much radiation is the average person exposed to annually from all sources?
620 mrem, or 6.2 mSv
2.26 If high doses of radiation can be harmful, what is the rationale behind radiation therapy for people with cancer?
An intense short-term dosage will swiftly destroy tissue in the exposed area. Cell types that are rapidly dividing, such as white blood cells (WBCs) and cancer cells, are killed off more quickly than others. Therefore, this intense radiation is very useful in the treatment of some cancers.
2.27 A routine dental exam often includes four bite-wing X-rays, exposing a patient to a total of 5 mrem of radiation. Would this cause radiation sickness in the patient? If so, what would be the effects?
No; you would have to receive 4000 sets of X-rays before observing a clinical effect (which starts to occur at 20,000 mrem). This is more than a dental technician would likely take in a day.
2.28 How many simultaneous chest X-rays would a person have to endure to have an exposure of 20,000 mrem, causing a temporary decrease in white blood cells? Based on this example, is one chest X-ray harmful?
-Dose from a single chest X-ray: Approximately 10 mrem
-Dose cited as causing temporary leukopenia: 20,000 mrem
20,000 mrem ÷ 10 mrem/X-ray = 2,000 times chest X-ray
A single, standard chest X-ray is not considered harmful (at least, it is far from the level that would cause a decrease in white blood cells).
2.6 Writing Nuclear Decay Equations Write a nuclear decay equation for americium-241 undergoing alpha decay.
Americium (Am) has a mass number of 241, and when it loses an alpha particle, that alpha particle has a mass of 4, of which 2 are protons, so the resulting atom will have a mass number of 237 and an atomic number of 93.
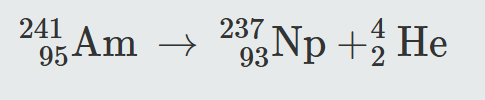
2.29 Write a balanced nuclear equation for the decay of each of the following:
carbon-14 undergoing beta decay
polonium-212 undergoing alpha decay
copper-66 undergoing beta decay
carbon-11 undergoing positron emission
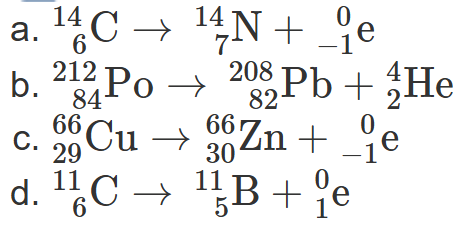
2.30 Write a balanced nuclear equation for the decay of each of the following:
thorium-232 undergoing alpha decay
strontium-92 undergoing beta decay
nitrogen-13 undergoing positron emission
californium-251 undergoing alpha decay

2.31 Write a balanced nuclear equation for the beta decay of cobalt-60, used in brachytherapy.
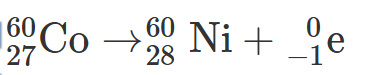
2.32 Write a balanced nuclear equation for the production of iodine-131, used in the treatment of some cancers, from the beta decay of tellurium-131.
