Evidence for electronic configuration
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
Atomic emission spectra shows…
…quantum shell numbers
How to obtain emission spectra
Use discharge tube
Electric current passed through a sample of gas
Electrical energy causes molecules to split into atoms
Electrons given energy, get promoted to higher energy levels
Electrons fall back down, emit light of characteristic frequencies
Shown as lines of colour
If there were no fixed quantum shells, there would be a continuous spectrum of colour
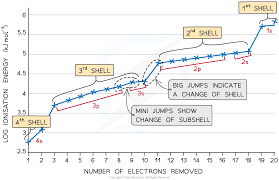
Successive ionisation energy graph and explanation
Jumps in ionisation energy between shells (not subshells) of the same atom due to electron being removed from a different shell
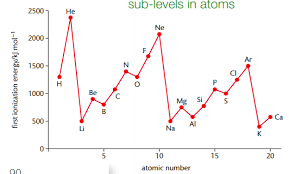
First ionisation energy graph and explanation
Jumps in first ionisation energy of successive elements across a period, shows presence of subshells (eg electron removed from a p subshell, not an s subshell)