Intermolecular Forces and States of Matter
1/501
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
502 Terms
What characterizes the particle arrangement in solids?
Solids have highly ordered assemblies of particles in close contact with one another.
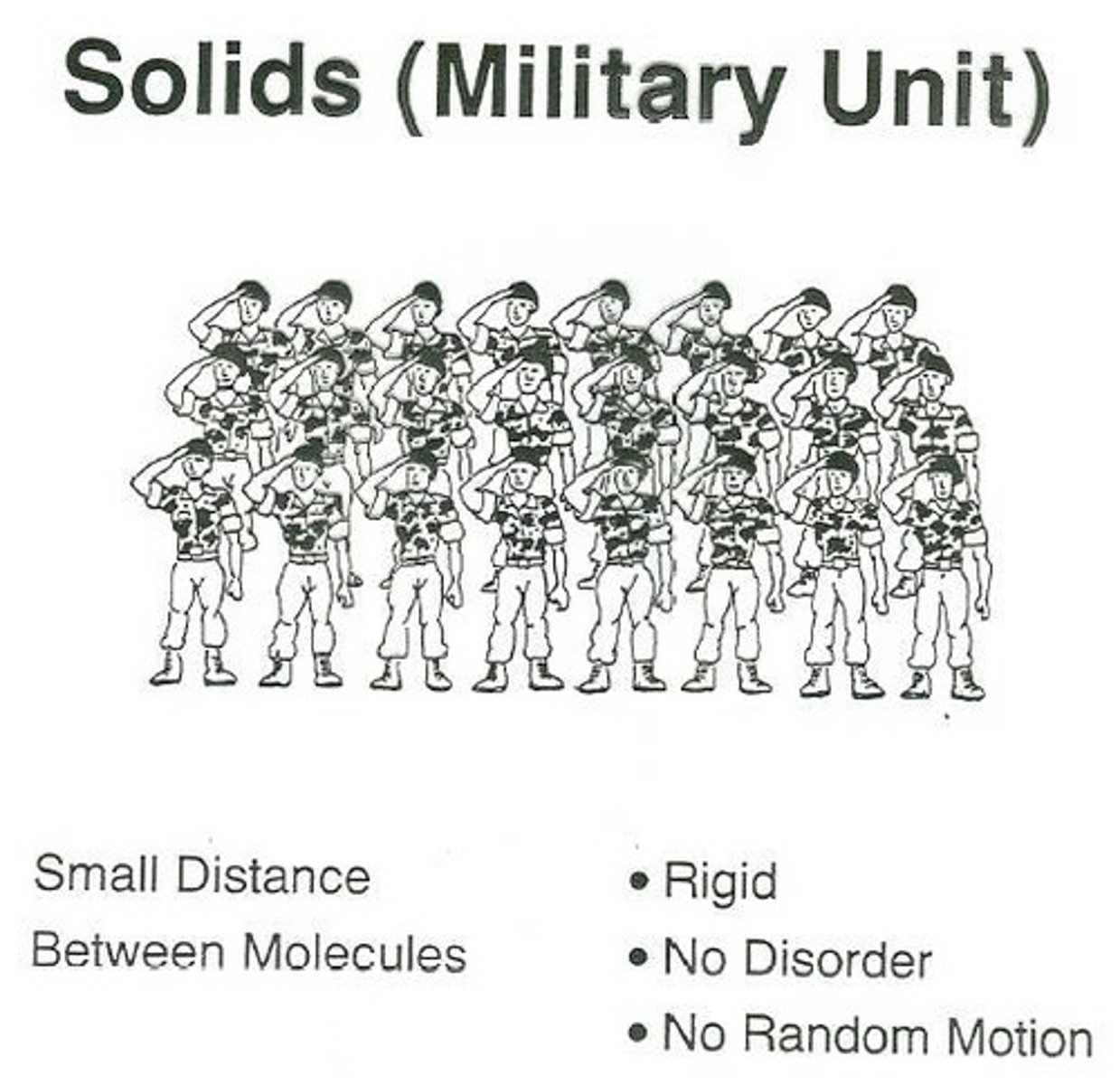
How does particle motion in solids compare to liquids and gases?
In solids, the motion of particles is very limited, while in liquids it is less restrained, and in gases, particles move randomly.
What are the key properties of solids?
Solids maintain both shape and volume, are virtually incompressible, do not flow, and diffusion is extremely slow.
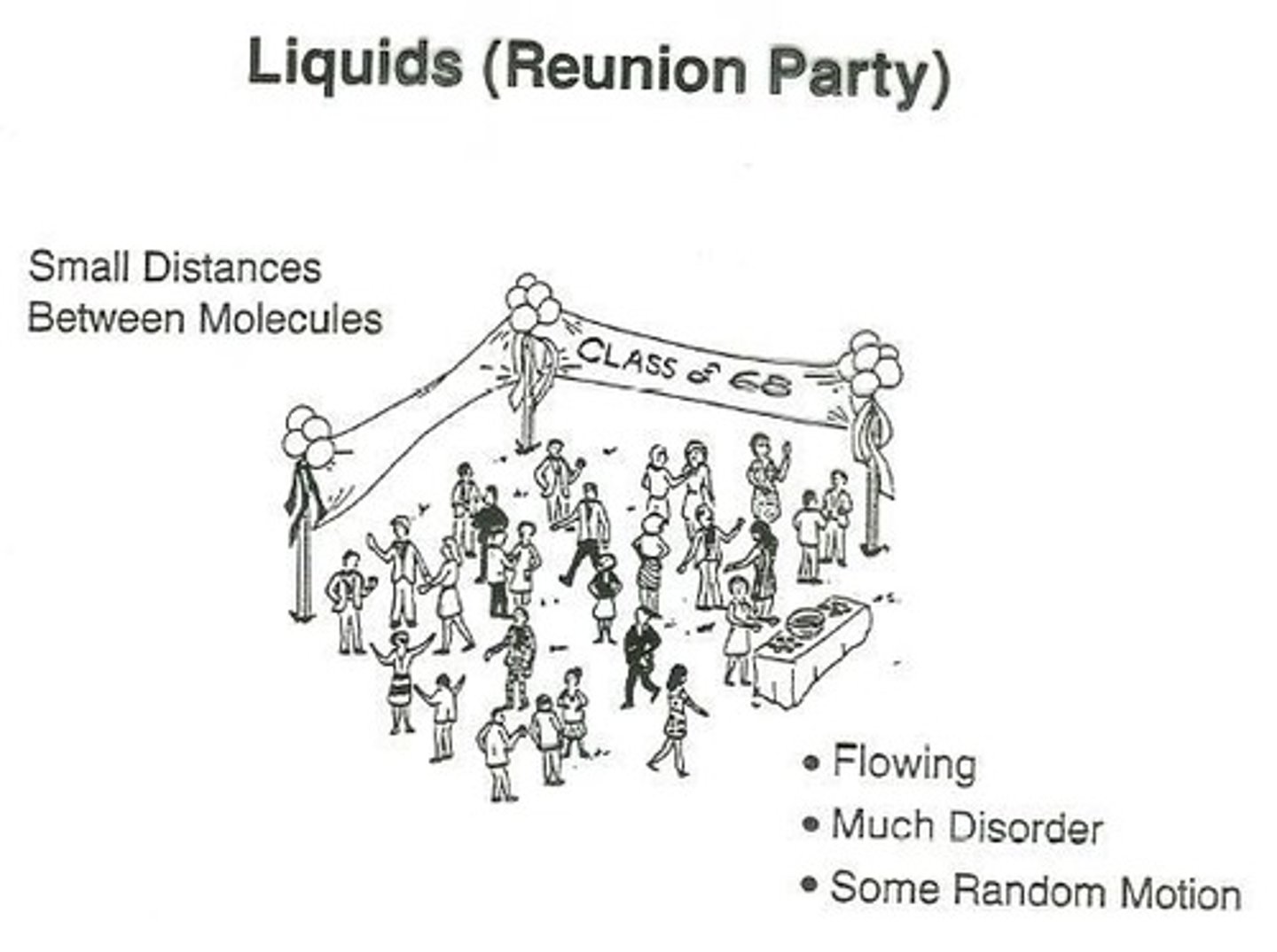
What properties do liquids exhibit?
Liquids maintain volume but not shape, adopt the shape of the container, do not expand to fill the container, are virtually incompressible, flow easily, and diffusion occurs slowly.
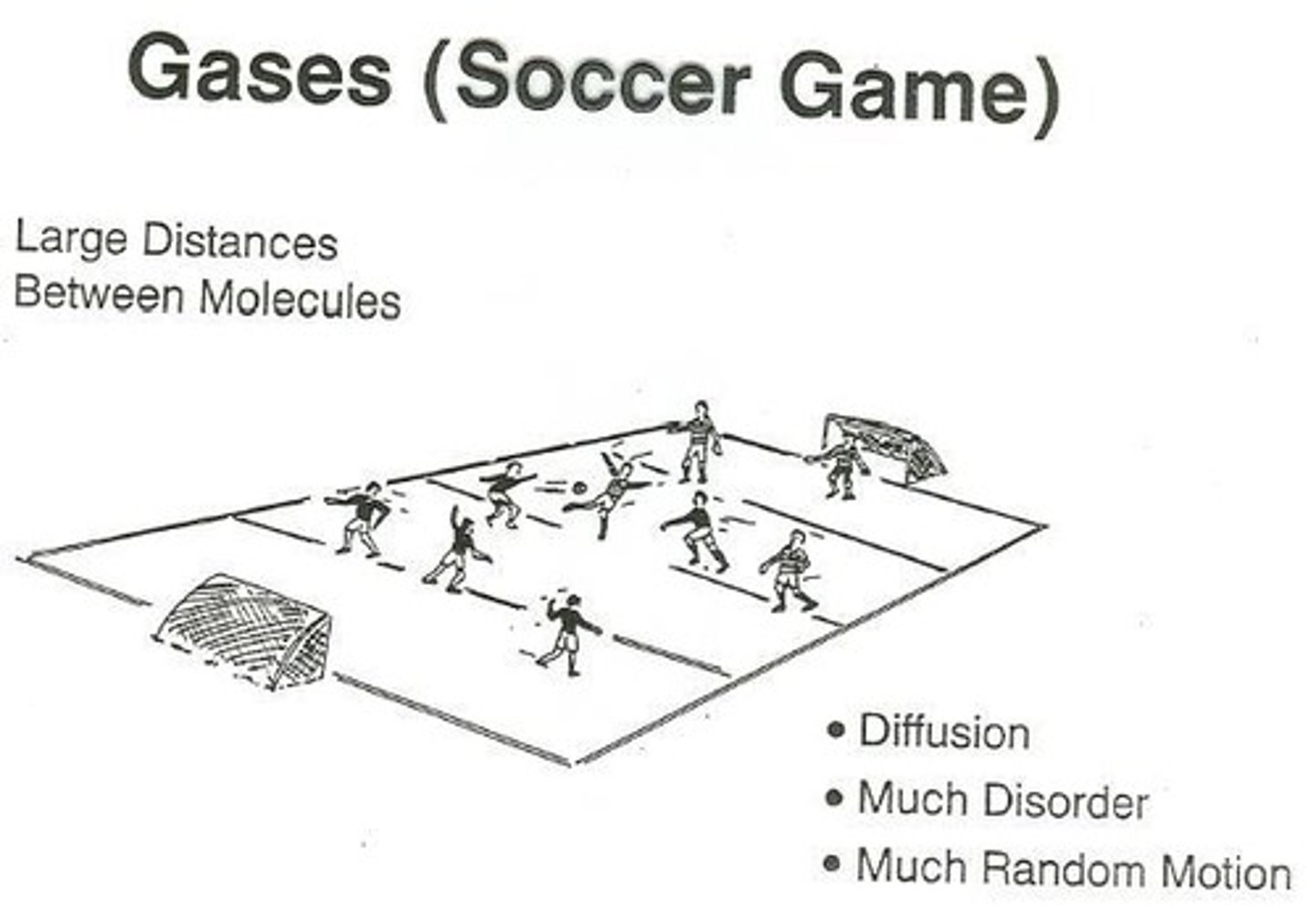
What characteristics define gases?
Gases maintain neither volume nor shape, adopt both volume and shape of the container, flow readily, and diffusion is rapid.
What is required for a substance to change from a higher-order to a lower-order state of matter?
Breaking bonds between the particles requires energy, and stronger bonds require more energy to break.
What are intermolecular forces?
Attraction forces acting between molecules, which are generally weaker than intramolecular bonding forces.
What are the main types of intermolecular forces?
Ion-dipole interactions, dipole-dipole interactions, hydrogen bonding, and London dispersion forces.
What are van der Waals forces?
Dipole-dipole and London dispersion forces are collectively referred to as van der Waals forces.
What is the significance of ion-dipole interactions?
They are important in solutions of ionic compounds in polar liquids, such as water, facilitating dissolution and electrolytic dissociation.
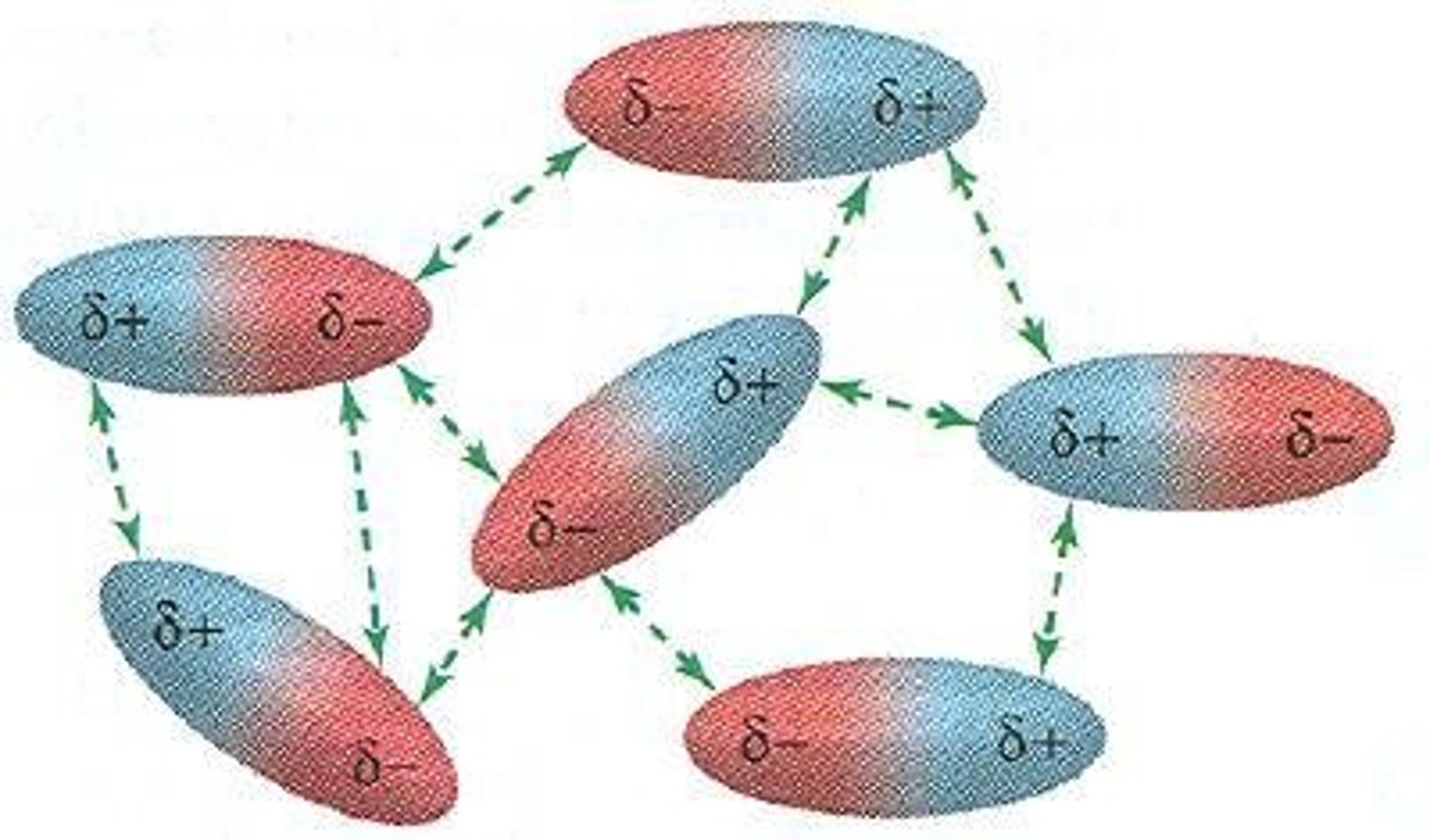
What are dipole-dipole interactions?
Attractive interactions between opposite charges in polar covalent compounds, which are weaker than ion-dipole interactions.
How is the dipole moment (μ) calculated?
μ = Q × r, where Q is charge magnitude and r is the distance between charges.
What is the SI unit for dipole moment?
The SI unit for dipole moment is Debye (D), where 1 D = 3.336×10-30 C·m.
How does dipole moment affect intermolecular forces?
The larger the dipole moment, the stronger the dipole-dipole intermolecular forces in a substance.
How does boiling point relate to molecular weight and intermolecular forces?
Boiling point typically increases with molecular weight but strongly depends on the strength of intermolecular forces; stronger forces result in higher boiling points.
What is the relationship between polarity and boiling point?
More polar substances, with larger dipole moments, boil at higher temperatures than less polar or non-polar substances.
What is a hydrogen bond?
A bond X--H⋅⋅⋅Y that forms between two electronegative atoms X and Y via a hydrogen atom, typically involving O, N, or F.
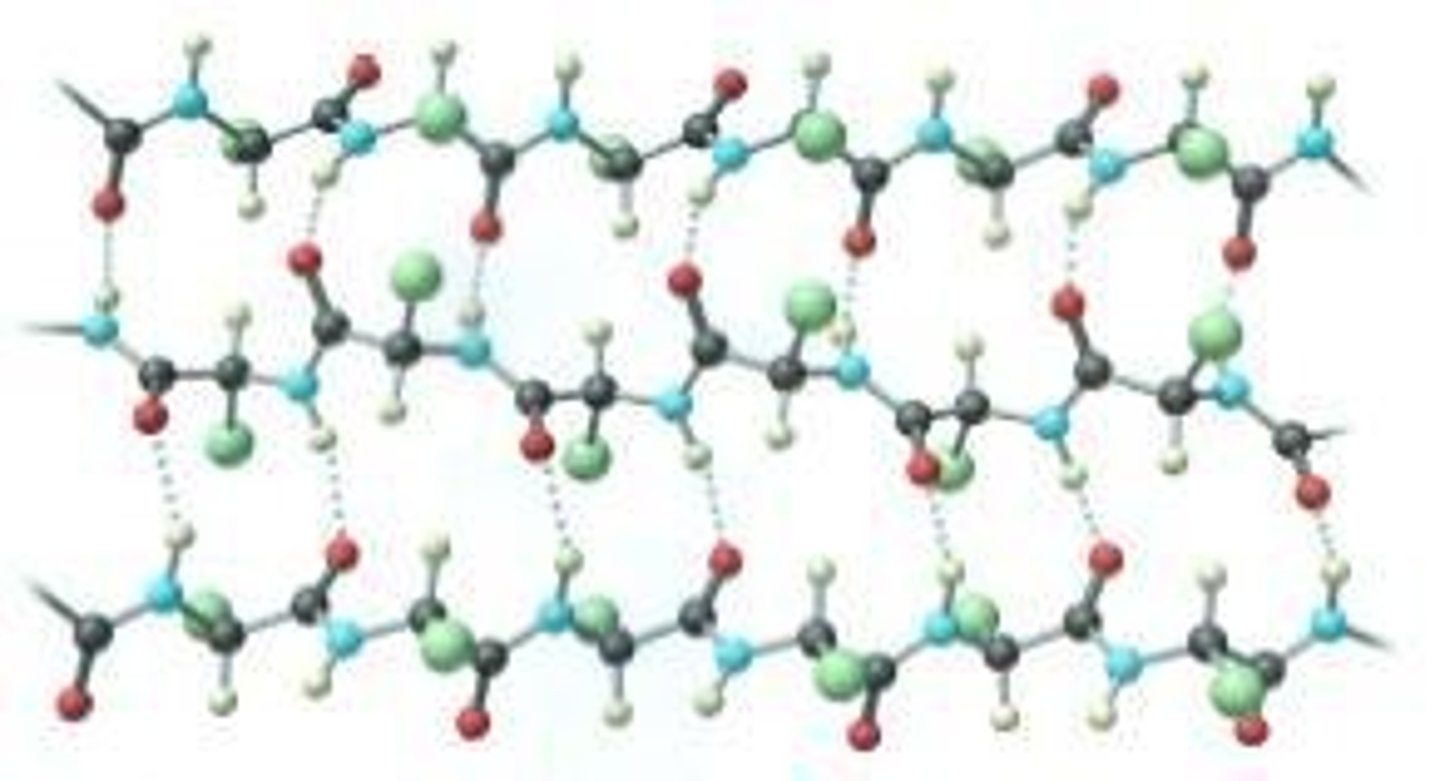
What role do lone electron pairs play in hydrogen bonding?
Lone electron pairs of electronegative atoms (O, N, F) are involved in forming hydrogen bonds.
What is the effect of ionic compounds on dipole moment?
Typical ionic compounds possess very large dipole moments, leading to high melting and boiling points.
What is the typical dipole moment of NaCl?
The dipole moment of NaCl is 9.0 D.
What happens to the strength of intermolecular forces as the dipole moment increases?
As the dipole moment increases, the strength of intermolecular forces also increases.
What is the diffusion rate in solids compared to liquids and gases?
Diffusion in solids is extremely slow, in liquids it occurs slowly, and in gases it is rapid.
What is hydrogen bonding in water molecules?
A moderate-to-strong interaction where the oxygen atom has a partial negative charge and hydrogen atoms have partial positive charges, allowing for attraction between water molecules.
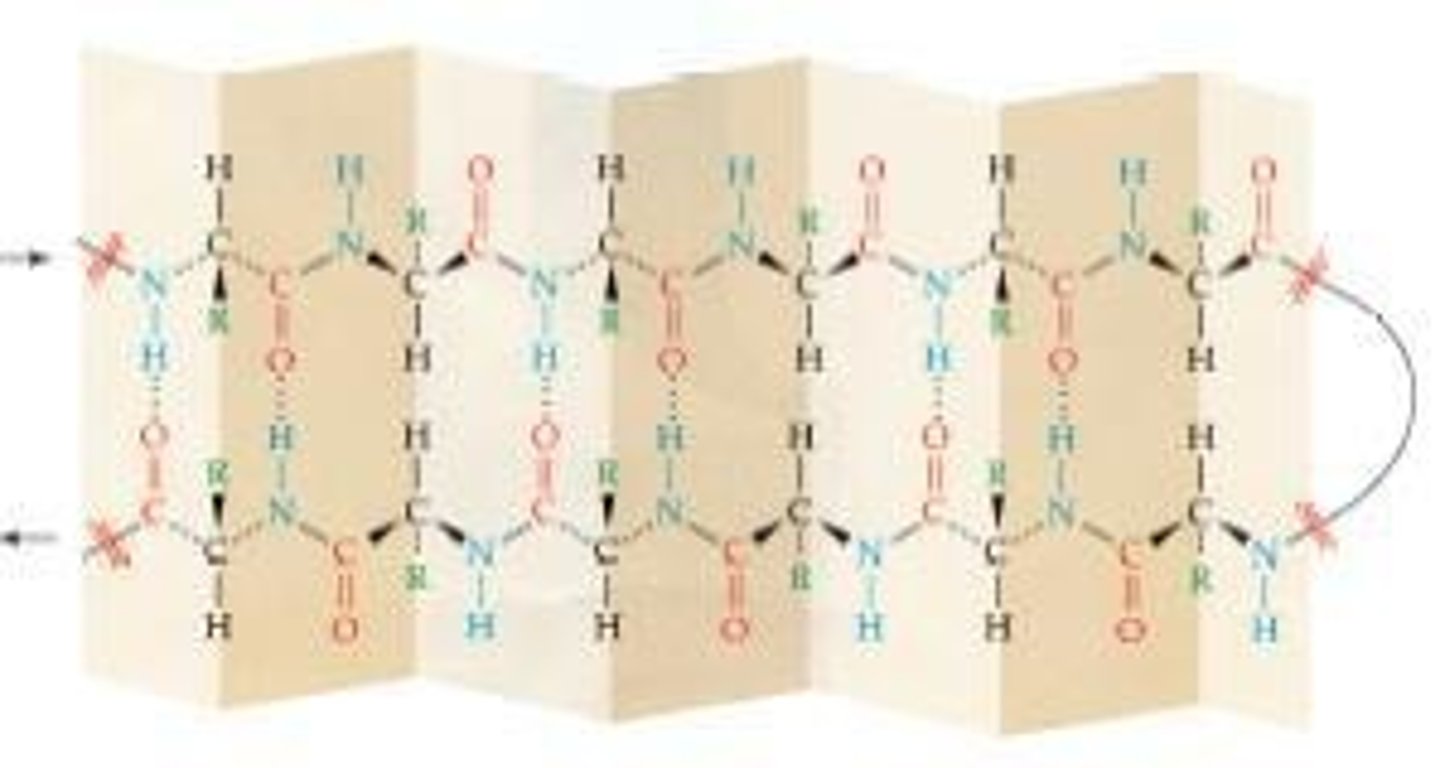
What role does hydrogen bonding play in biological molecules?
Hydrogen bonding is crucial for the structure of many biological molecules, such as proteins and DNA.
How does hydrogen bonding affect the boiling points of substances?
Hydrogen bonding leads to abnormally high boiling points for substances like H2O, HF, and NH3 compared to other molecules of similar molecular weight.
What is the boiling point of water at room temperature?
100 °C.
Why is water a liquid at room temperature while H2S, H2Se, and H2Te are gases?
Water's hydrogen bonding results in a higher boiling point than these heavier molecules, despite their larger molecular weights.
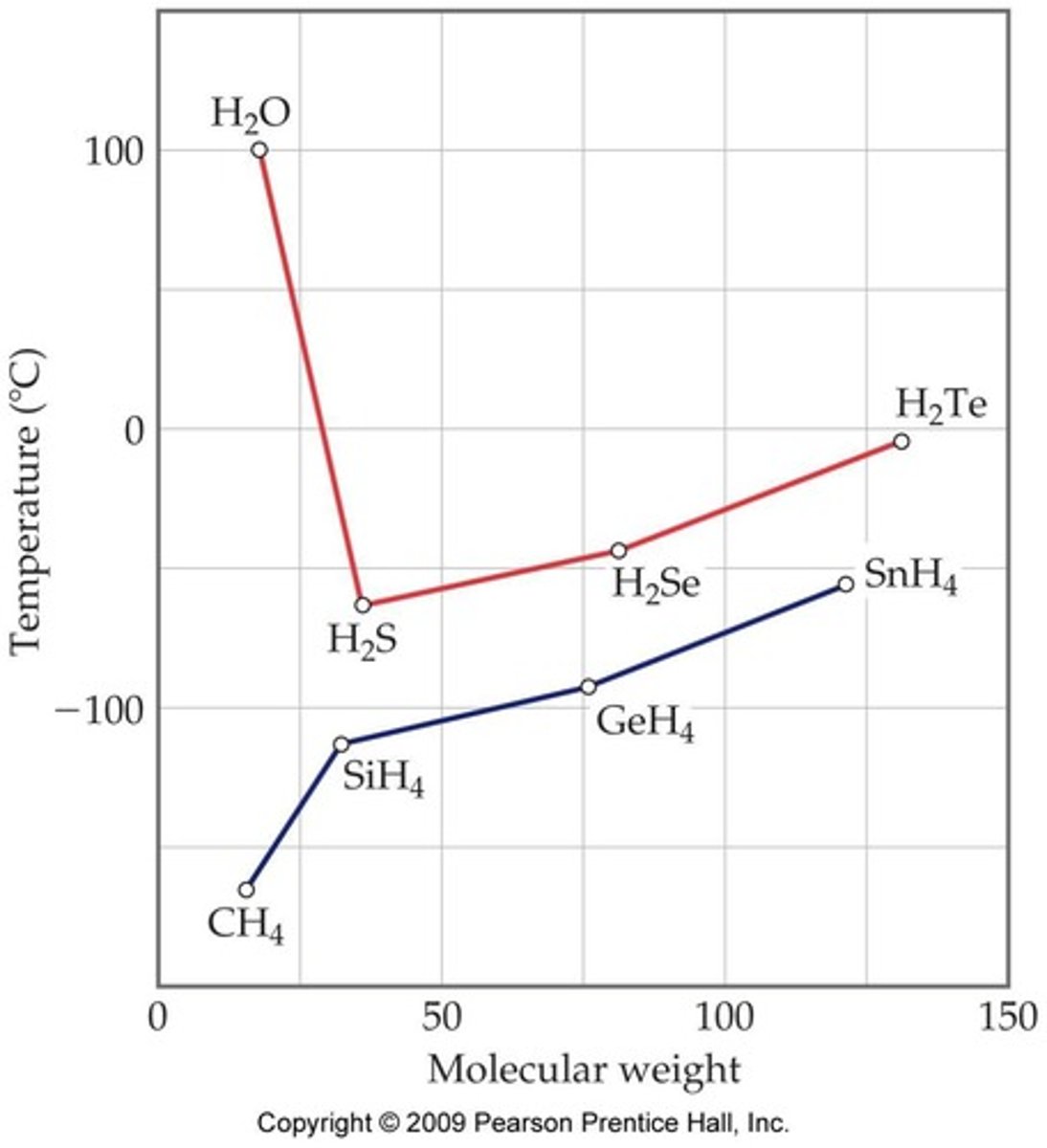
What are London Dispersion Forces?
Weak attractive forces that exist in all substances, particularly significant in non-polar molecules.
In which types of molecules are London Dispersion Forces most important?
Non-polar molecules, such as H2, O2, CH4, CCl4, and CO2.
What determines the magnitude of dispersion forces in non-polar compounds?
The polarizability of the molecules; higher polarizability leads to a greater induced dipole moment.
How does the size and weight of molecules affect polarizability?
Polarizability generally increases with increasing size and weight of the molecules, resulting in stronger dispersion forces.
What is the boiling point of F2, Cl2, Br2, and I2?
F2: 85 K, Cl2: 238.6 K, Br2: 331.9 K, I2: 457.5 K.
What is the melting point of F2, Cl2, Br2, and I2?
F2: 53.3 K, Cl2: 172.2 K, Br2: 265.9 K, I2: 386.7 K.
What is the physical state of I2 at room temperature?
Solid.
How does the shape of molecules affect their polarizability?
Linear molecules are usually polarized easier than branched molecules, resulting in stronger dispersion forces for linear molecules.
What is the first step in predicting the physical properties of a molecular substance?
Assess the impact of intermolecular forces (interactions) involved.
What are the major intermolecular forces present in H2S?
Dipole-dipole interactions and dispersion forces.
What intermolecular forces are present in Ne?
Dispersion forces only.
What intermolecular forces are present in CH3OH?
Hydrogen bonding, dipole-dipole interactions, and dispersion forces.
What is the classification of hydrogen bonding in terms of energy?
Hydrogen bonding is classified as a moderate-to-strong interaction with energy ranging from 5-25 kJ/mol.
What is the significance of the lone electron pairs on the oxygen atom in water?
They allow the oxygen atom to donate pairs to hydrogen atoms of neighboring water molecules, facilitating hydrogen bonding.
What is the relationship between molecular weight and boiling point in substances without hydrogen bonding?
Typically, boiling point increases with molecular weight.
What is the effect of hydrogen bonding on the boiling point of H2O compared to other substances?
H2O has an abnormally high boiling point due to hydrogen bonding, unlike heavier substances that are gases.
How do intermolecular forces influence the physical state of a substance?
The type and strength of intermolecular forces determine whether a substance is solid, liquid, or gas at a given temperature.
What is the effect of increasing molecular weight on dispersion forces?
Increasing molecular weight generally leads to stronger dispersion forces due to higher polarizability.
What types of intermolecular forces are present in CH3OH?
H-bonding, dipole-dipole interactions, and dispersion forces.
What intermolecular forces are present in CH4?
Dispersion forces only.
How does the boiling point of substances change with size and intermolecular forces?
The boiling point increases as the size increases and as the intermolecular forces become stronger.
Arrange the following substances in order of increasing boiling point: Ne, CH4, H2S, CH3OH.
Ne < CH4 < H2S < CH3OH.
What is viscosity and how is it related to molecular attraction?
Viscosity is related to the ability of molecules in a liquid to move with respect to one another; stronger attraction forces increase viscosity.
How does molecular weight affect viscosity?
Viscosity normally increases with molecular weight.
What is surface tension?
Surface tension is the resistance of a liquid to spread out and increase its surface area due to imbalance of intermolecular forces at the surface.
What is cohesion in liquids?
Cohesion is the attraction of the same kind of molecules in a liquid.
What is adhesion in liquids?
Adhesion is the attraction of liquid molecules to the molecules on the surface of another substance.
What causes the U-shaped (concave) meniscus in water?
The adhesion of water to glass is stronger than the cohesion of water molecules.
What causes the convex meniscus in mercury?
The adhesion of mercury to glass is weaker than the strong cohesive forces between mercury molecules.
What is capillary action?
Capillary action is the rise of liquids up very narrow tubes due to adhesive forces between the liquid and the walls of the tube.

What are phase changes?
Processes in which the physical form of a substance changes, but not its chemical identity.
What is fusion in terms of phase changes?
Fusion (melting) is the phase change from solid to liquid.
What is freezing in terms of phase changes?
Freezing is the phase change from liquid to solid.
What is sublimation?
Sublimation is the phase change from solid to gas.
What is deposition?
Deposition is the phase change from gas to solid.
What is vaporization?
Vaporization is the phase change from liquid to gas.
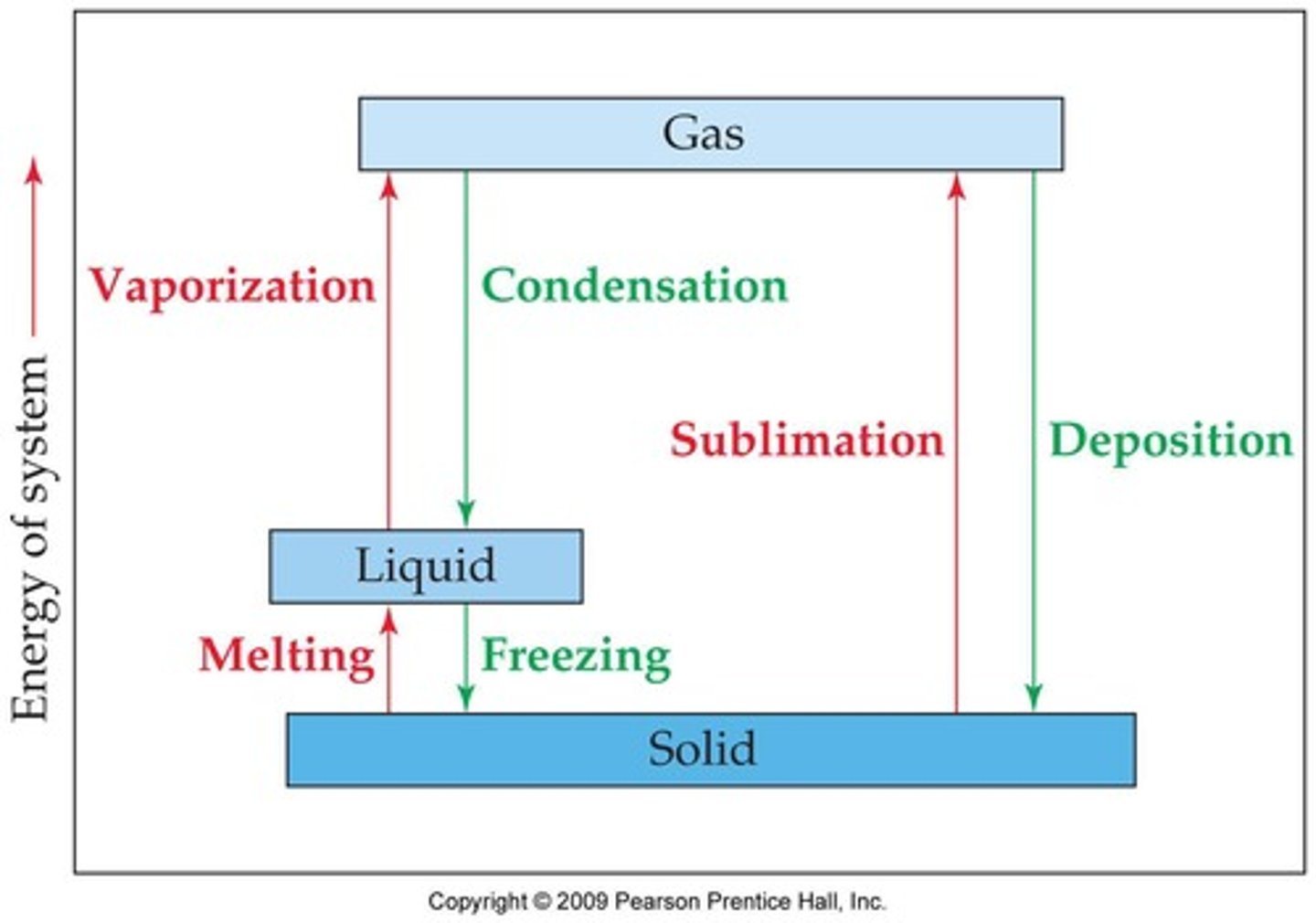
What is condensation?
Condensation is the phase change from gas to liquid.
How are energy changes related to phase changes?
Phase changes are always accompanied by changes in energy of the system, with opposite signs for reverse processes.
What is the significance of the melting point?
The melting point is the temperature at which a substance is in equilibrium between solid and liquid.
What occurs at the boiling point of a substance?
At the boiling point, the substance is in equilibrium between liquid and gas, and the temperature remains unchanged until all the liquid evaporates.
What is a heating curve?
A heating curve is a plot of the measured internal temperature of a substance as a function of the added heat.
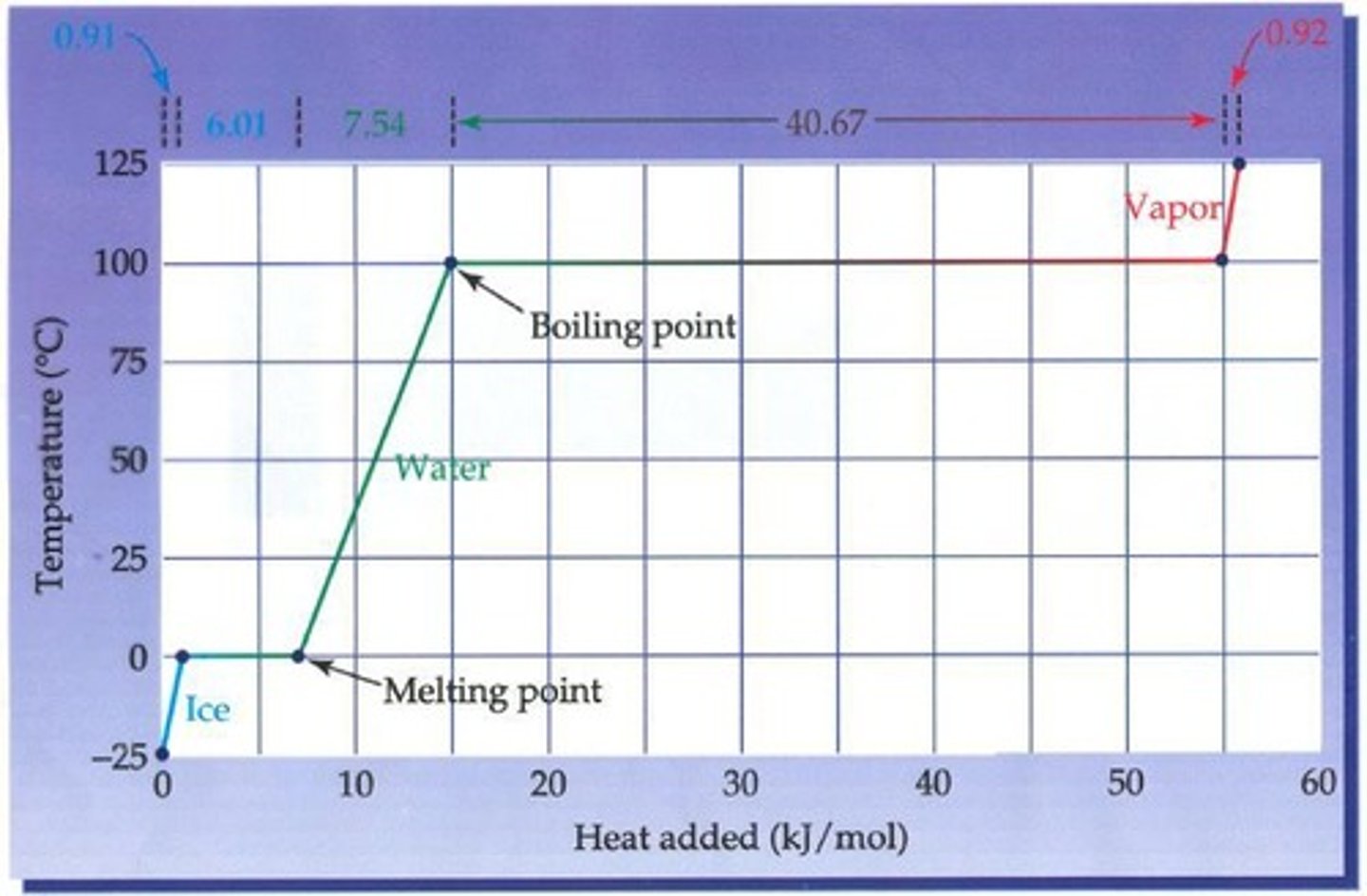
What happens to temperature at the melting point?
At the melting point, the temperature remains unchanged until all solid converts into liquid.
What happens to temperature at the boiling point?
At the boiling point, the temperature remains unchanged until all liquid is evaporated.
What is the enthalpy of fusion of ethanol?
5.02 kJ/mol
What is the enthalpy of vaporization of ethanol?
38.56 kJ/mol
What is the specific heat of solid ethanol?
0.97 J/g·K
What is the specific heat of liquid ethanol?
2.3 J/g·K
What is the formula to calculate heat when there is no phase change?
ΔH = (Specific heat) × (Mass of substance) × (ΔT)
What is the formula to calculate heat during a phase change?
ΔH = (ΔH phase change) × (Moles of substance)
What are the segments of the heating curve for ethanol when converting from solid to vapor?
Segments include heating solid ethanol, fusion, heating liquid ethanol, and vaporization.
What is the temperature range for the solid phase of ethanol?
Below -114°C
At what temperature does ethanol boil?
78°C
How do you calculate the moles of ethanol from mass?
Moles = (Mass of ethanol) / (Molar mass of ethanol), where molar mass = 46.0 g/mol.
What is the total enthalpy change formula for converting ethanol from solid at -155°C to vapor at 78°C?
ΔH = ΔH1 + ΔH2 + ΔH3 + ΔH4.
What does ΔH1 represent in the heating curve for ethanol?
Heating solid ethanol from -155°C to -114°C.
What does ΔH2 represent in the heating curve for ethanol?
Fusion of solid ethanol at -114°C.
What does ΔH3 represent in the heating curve for ethanol?
Heating liquid ethanol from -114°C to 78°C.
What does ΔH4 represent in the heating curve for ethanol?
Vaporization of ethanol at 78°C.
What is the total heat required to convert 42.0 g of ethanol from -155°C to vapor at 78°C?
Approximately 60 kJ.
What is the significance of vapor pressure in liquids?
It is the pressure exerted by vapor in equilibrium with its liquid at a constant temperature.
Does vapor pressure depend on the volume of the container?
No, it is constant at a given temperature and does not depend on the volume.
What is the relationship between temperature and vapor pressure?
Vapor pressure increases with temperature due to increased molecular escape from the liquid.
What is the change in temperature (ΔT) when heating liquid ethanol from 35°C to 78°C?
43°C (or 43 K).
How much heat is required to raise the temperature of 42.0 g of liquid ethanol from 35°C to 78°C?
Approximately 4.15 kJ.
What is the total enthalpy change when converting 42.0 g of ethanol at 35°C to vapor at 78°C?
Approximately 39 kJ.
What is the molecular weight of ethanol?
46.0 g/mol.
What happens to the temperature during a phase change?
The temperature remains constant while heat is added.
What does vapor pressure depend on?
Vapor pressure depends on the strength of intermolecular interactions in the substance.
How does the strength of intermolecular forces affect vapor pressure?
The weaker the intermolecular attractive forces, the higher the vapor pressure of the liquid.
What is the relationship between intermolecular forces and boiling point?
The weaker the intermolecular forces, the lower the boiling point of the liquid.
What term is used to describe substances that evaporate readily and have high vapor pressure?
Volatile.
Compare the vapor pressure of diethyl ether to that of ethanol and water at the same temperature.
Diethyl ether has a much higher vapor pressure than ethanol and water due to weaker intermolecular forces.
What happens when the vapor pressure of a liquid equals atmospheric pressure?
The liquid boils.