Acid base disorders & Blood gas analysis
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
47 Terms
Acid (H+) Definition:
substance that can yield a hydrogen ion when dissolved in H2O
Base Definition:
(bicarbonate) or OH- (hydroxide ion) - substance that can ACCEPT H+
pH Definition:
inversely proportional to [H+]
Critical pH
7.35 - 7.45 in the human body
pH below 7.35 is an acidemia, and a pH above 7.45 is an alkalemia
Neutral pH Definition:
7, on a scale of 1 to 14
Normal arterial blood pH Definition:
@ 37°C is 7.40 ± 0.05
Normal venous blood pH Definition:
@ 37°C is 7.37 ± 0.05
Metabolically Produced Acids
Volatile acid = CO2 (~20 moles produced each day by normal metabolism)
Nonvolatile acids: non CO2 (~100 mmoles/day by normal metabolism)
Nonvolatile acids
acids derived from sources other than CO2
uric acid, phosphoric acid, sulfuric acid, acetoacetic acid, etc.
cannot be removed through lungs and must be excreted through the kidney

Types of Buffer Systems
Bicarbonate/carbonic acid buffer system: H(+) + HCO3 ⇌ H2CO3 ⇌ CO2 + H2O
Protein buffer: Most circulating proteins carry a net negative charge capable of binding H+
Albumin: accounts for about 95% of the buffering capacity of proteins
Phosphate buffer: HPO4^2- & H2PO4 - in plasma and RBC pH balance (2,3 DPG)

Acid-Bicarbonate Buffer System
When H⁺ increases in the body, it combines with HCO₃⁻ (bicarbonate) to form H₂CO₃ (carbonic acid) → breaks down into CO₂ and H₂O.
system neutralizes excess acidity through reactions that involve sodium bicarbonate (NaHCO₃) → allowing bicarbonate to buffer excess H⁺
prevents significant pH changes
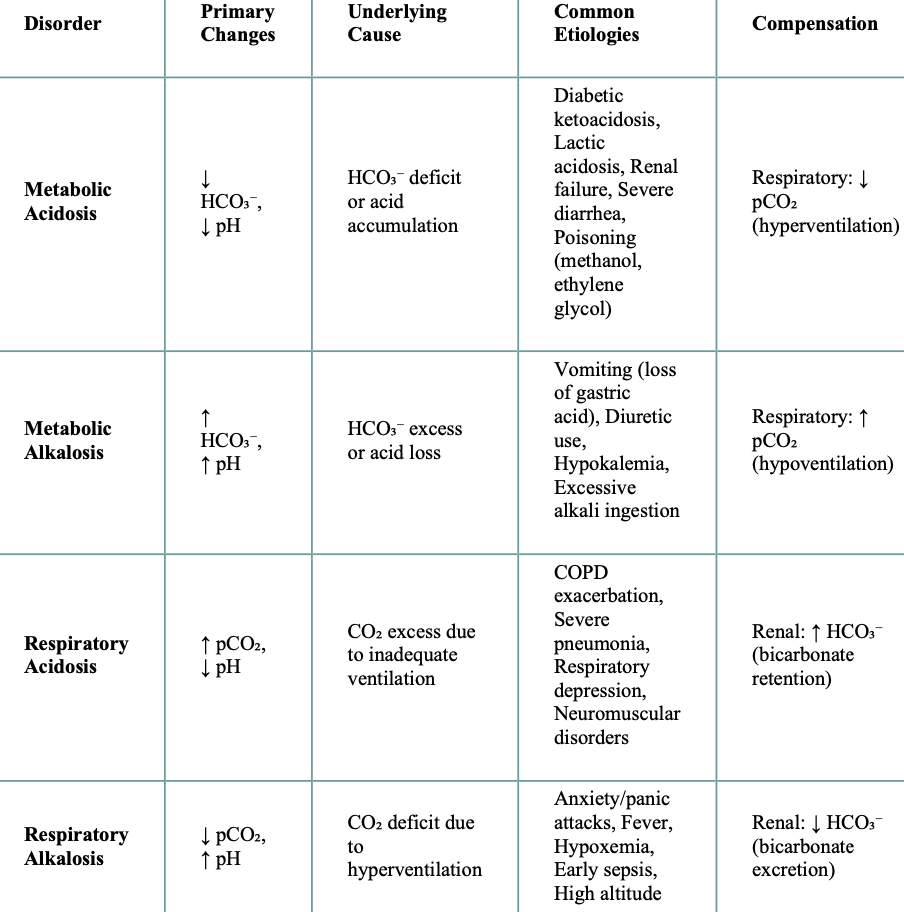
Acidosis vs Alkalosis
Acidosis: CO₂ → More H₂CO₃ forms → More H⁺ is released → pH drops (acidosis)
Alkalosis: CO₂ → Less H₂CO₃ forms → Less H⁺ is available → pH rises (alkalosis)
Controlling Blood pH - Buffers
pH imbalance due to changes in HCO₃⁻ → metabolic and primarily regulated by the kidneys
pH imbalance due to changes in CO₂ → respiratory and primarily regulated by the lungs
Causes of Increased Acidity in the body: Respiratory
Chronic Obstructive Pulmonary Disease (COPD), severe asthma, pneumonia
Respiratory depression (opioids, sedatives)
Causes of Increased Acidity in the body: Metabolic
Metabolic causes:
Diabetic ketoacidosis (DKO)
Lactic acidosis
Kidney failure
severe diarrhea
Alcoholic ketoacidosis
Certain medications (aspirin overdose, metformin)
Starvation
Poisoning (ethylene glycol, methanol)
Causes of increased base/alkalinity in the body
Excessive vomiting (loss of stomach acid)
Overuse of antacids or baking soda
Certain medications (diuretics)
Hyperventilation (breathing too rapidly)
Severe dehydration
Milk-alkali syndrome
Predominance levels of CO2 content in blood
HCO₃⁻ (Bicarbonate) ~ 90-95%
Dissolved CO₂ (Physically dissolved gas) ~ 5%
H₂CO₃ (Carbonic Acid) <1
Measuring bicarbonate levels provides the most accurate total CO₂ content in the blood.
H2CO3 is unstable
Respiratory vs Metabolic Control
Lungs regulate CO2 levels through ventilation (Fast), while kidneys manage bicarbonate and hydrogen ion balance (slow).
Lungs (Respiratory Control)
Control CO2 (acid) levels through ventilation - fast response
Hyperventilation: increases CO2 elimination and raises pH
Ex: In response to diabetic ketoacidosis
Hypoventilation: decreases CO2 elimination and lowers pH
Hyperventilation
A physiological condition where increased breathing rate leads to excessive elimination of CO2, resulting in a rise in blood pH. It often occurs in response to metabolic disturbances like diabetic ketoacidosis.
Hypoventilation
A condition characterized by decreased breathing rate or depth, leading to elevated CO2 levels and a lowered blood pH.
Kidneys (Metabolic Control)
Control HCO3- (base) levels through reabsorption/excretion, slow response
Reabsorb HCO3 in the proximal convoluted tubule
Excrete H+ ions in exchange with Na+ (regulated by aldosterone)
Produce NH3 to buffer excess H+ and form NH4+ for excretion
Ex: Metabolic Alkalosis = excessive vomiting = H+ loss = HCO3- secretion by kidney
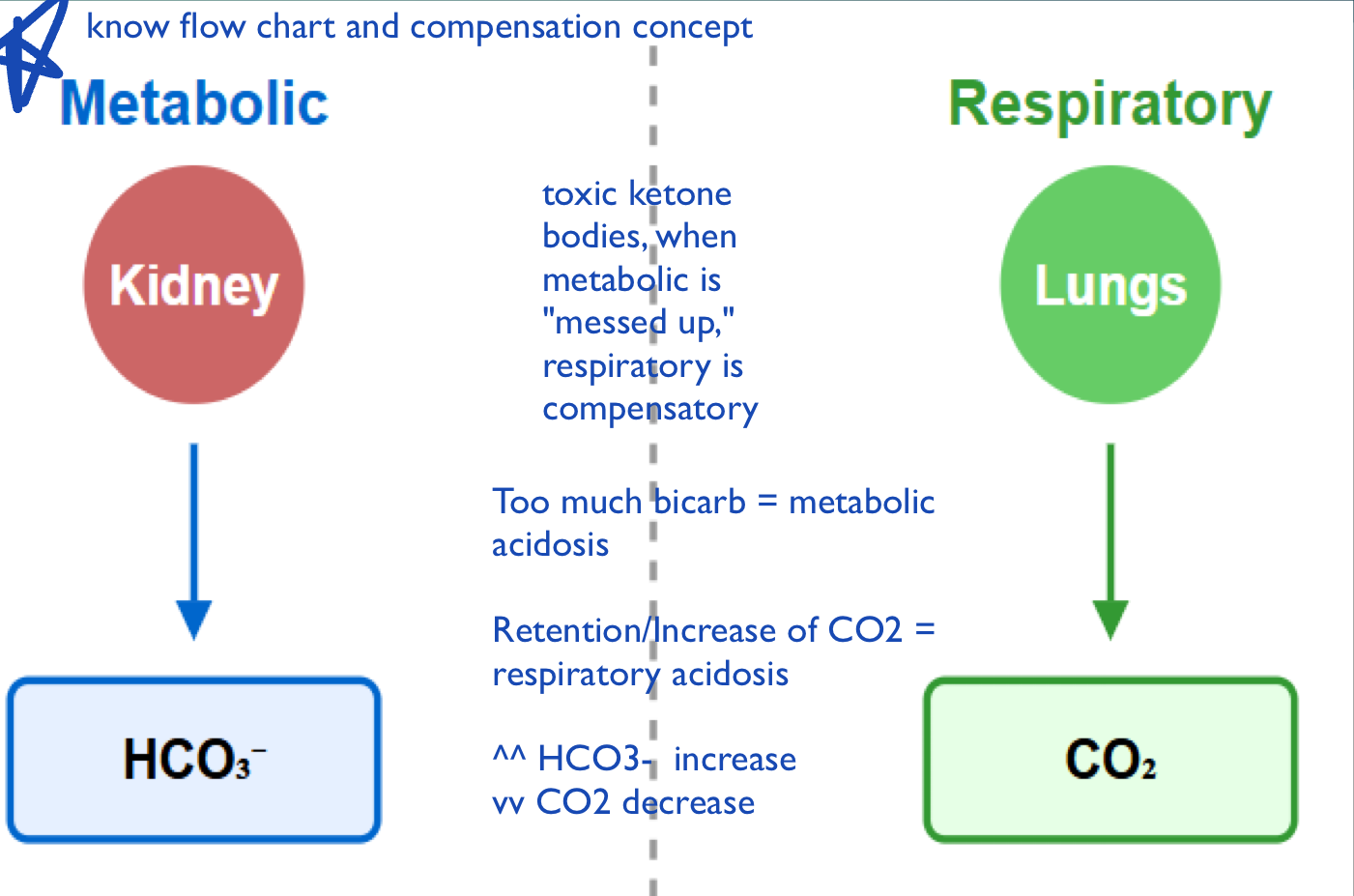
ROME
Respiratory Opposite, Metabolic Equal.
In respiratory disorders, pH and PCO2 move in opposite directions (acidosis: pH↓, PCO2↑; alkalosis: pH↑, PCO2↓).
In metabolic disorders, pH and HCO3- move together (acidosis: pH↓, HCO3-↓; alkalosis: pH↑, HCO3-↑).
Henderson-Hasselbalch Equation
pH = pK + log [HCO3-]/[H2CO3]
H2CO3 is measured as dissolved CO2 (PCO2)
Normal ratio of base to acid is approximately 20:1
CO2 content = HCO3- + H2CO3 (where H2CO3 = PCO2 × 0.03)
![<ul><li><p>pH = pK + log [HCO3-]/[H2CO3]</p><ul><li><p> H2CO3 is measured as dissolved CO2 (PCO2)</p></li></ul></li><li><p>Normal ratio of base to acid is approximately 20:1</p></li><li><p>CO2 content = HCO3- + H2CO3 (where H2CO3 = PCO2 × 0.03)</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/9c42d10f-a570-4127-9cd2-28d040624032.png)
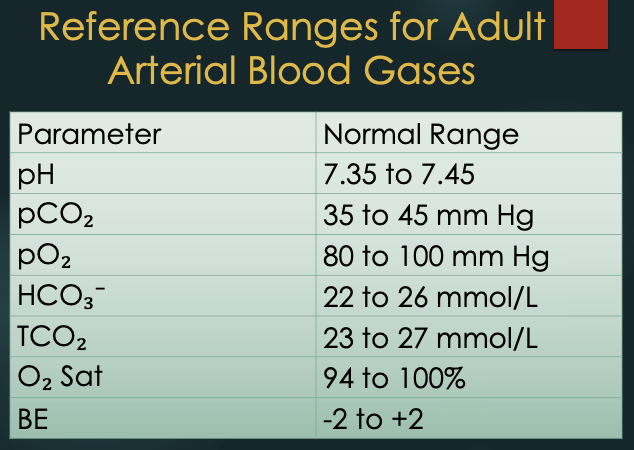
References Ranges for Adult Arterial Blood Gas (ABG)
Parameter, Normal Range
pH: 7.35 - 7.45
pCO2: 35 - 45 mm Hg
pO2: 80-100 mm Hg
HCO3-: 22 - 26 mmol/L
TCO2: 23-27 mmol/L
O2 saturation: 94 - 100%
BE: -2 to +2
Kidney Function
Absorb:
Bicarbonate for pH balance
Excrete:
H+ ions (in acidosis)
Bicarbonate (in alkalosis)
Phosphate (acid-base regulation)
Potassium for electrolyte balance
Generate:
Bicarbonate through buffering mechanisms
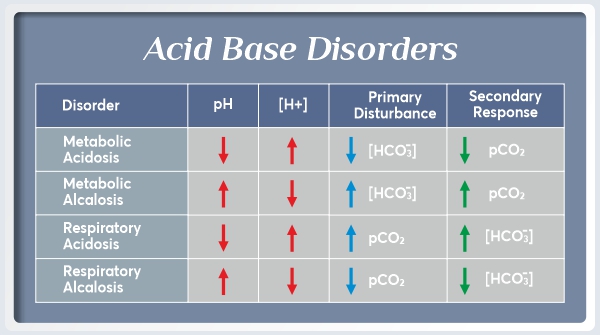
Clinical Causes of Acid-Base Disorders
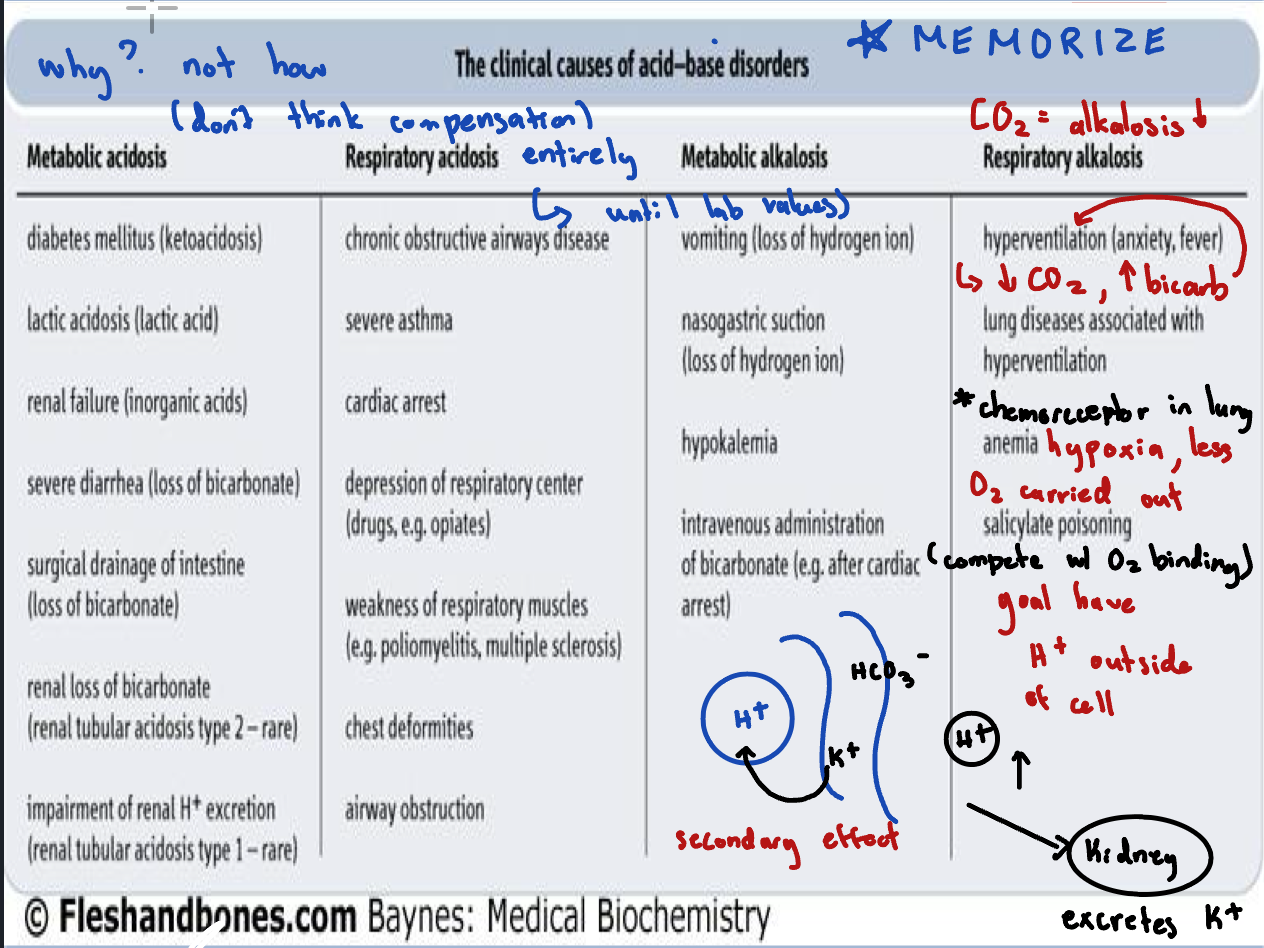
Primary Acid-Base Disorders
Respiratory acidosis: ↑ PCO2, ↓ pH (hypoventilation)
Respiratory alkalosis: ↓ PCO2, ↑ pH (hyperventilation)
Metabolic acidosis: ↓ HCO3-, ↓ pH (increased acid production or bicarbonate loss)
Metabolic alkalosis: ↑ HCO3-, ↑ pH (increased bicarbonate or loss of H+)
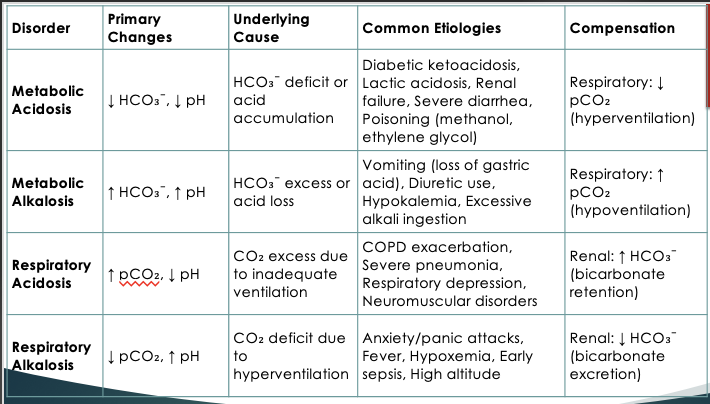
Respiratory acidosis:
If pH is low and bicarbonate (HCO₃⁻) is high, the elevated bicarbonate is a compensatory response, not the primary cause of the acidosis
Respiratory alkalosis:
CO₂ deficit (↓ pCO₂, ↑ pH), where the excessive loss of CO₂ leads to an increase in pH
Metabolic acidosis:
If pH is low AND pCO2 is low, CO2 (and therefore the lungs) is not the culprit – it is trying to compensate
Metabolic alkalosis:
If both pH and pCO₂ are high, the elevated pCO₂ is a compensatory response, not the primary cause of the disturbance.
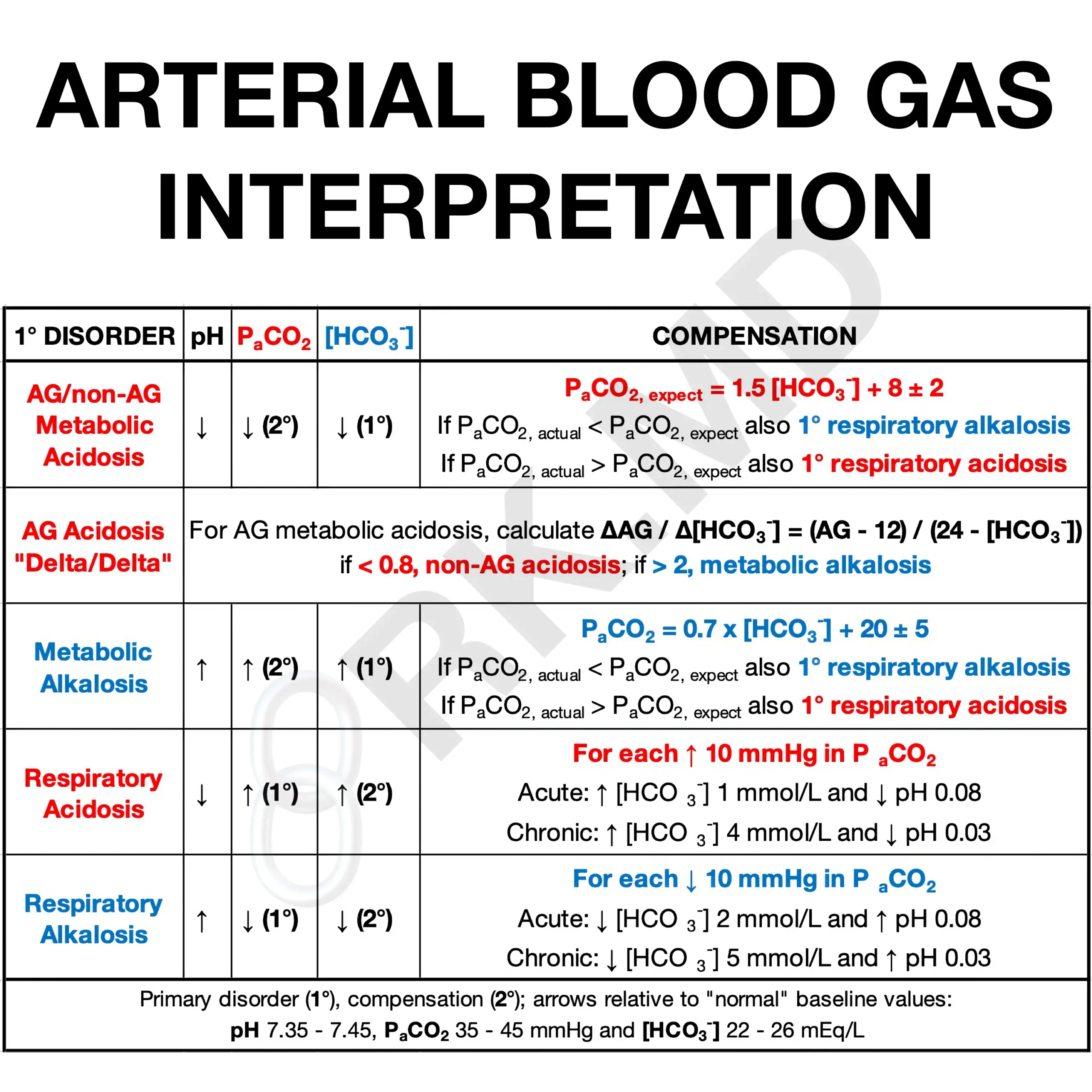
Imbalance Summary
The respiratory system compensates slowly for disturbances in the metabolic system and vice versa. Respiratory acidosis from CO2 retention prompts bicarbonate increase, while respiratory alkalosis from CO2 loss causes bicarbonate decrease. In metabolic acidosis, respiration increases to expel CO2, whereas in metabolic alkalosis, respiration slows to retain CO2.
Compensation Mechanism
If respiratory system is disturbed, metabolic system compensates (slowly)
If metabolic system is disturbed, respiratory system compensates (quickly)
Amount of Compensation:
No compensation: primary disorder with no compensatory response
Partial compensation: primary disorder with incomplete compensatory response
Complete compensation: primary disorder with full compensatory response and *pH returned to normal*
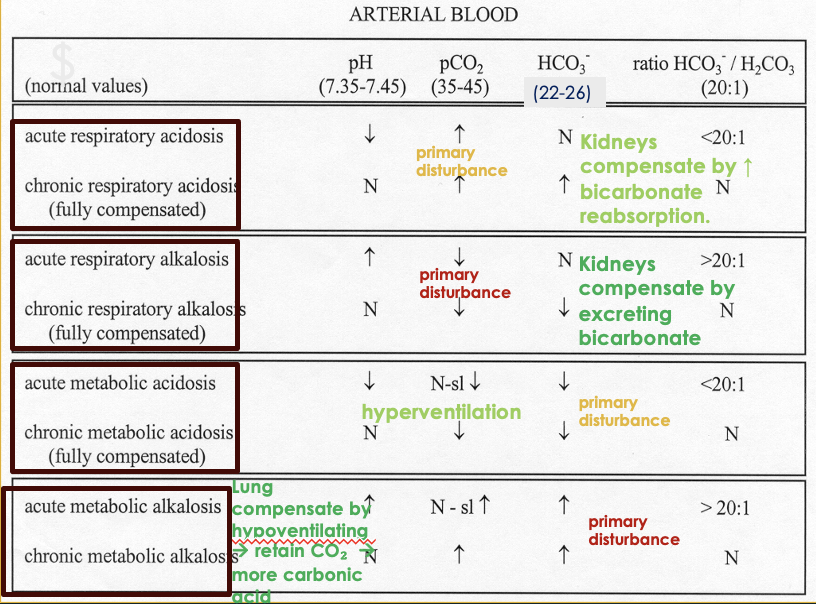
Diagnostic Criteria for Acid-Base Disorders
If pH is low and PCO2 is low: metabolic acidosis with respiratory compensation
If pH is low and HCO3 is high: respiratory acidosis with metabolic compensation
If pH and PCO2 are both high: metabolic alkalosis with respiratory compensation
If pH is high and PCO2 is low: respiratory alkalosis with metabolic compensation
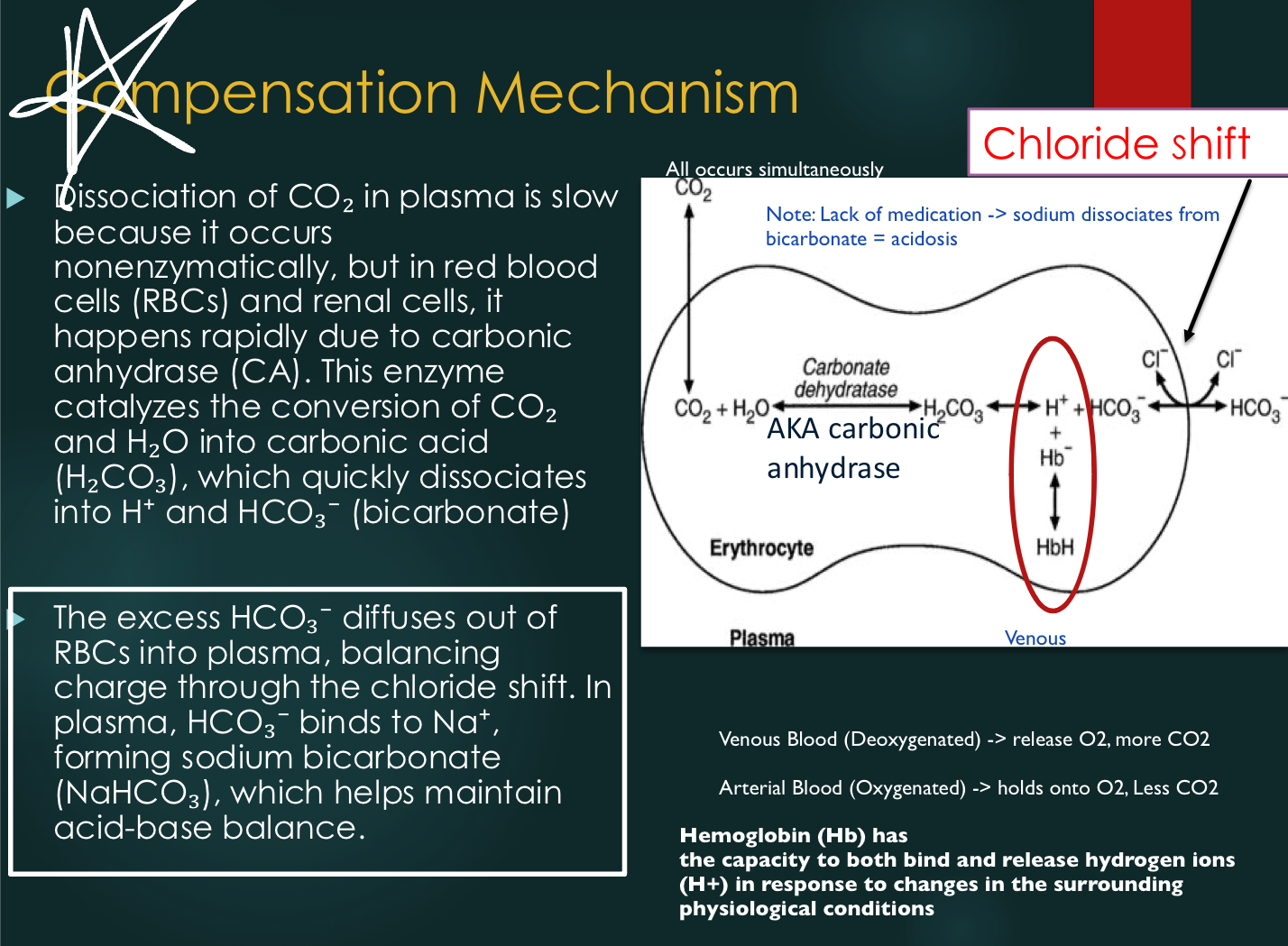
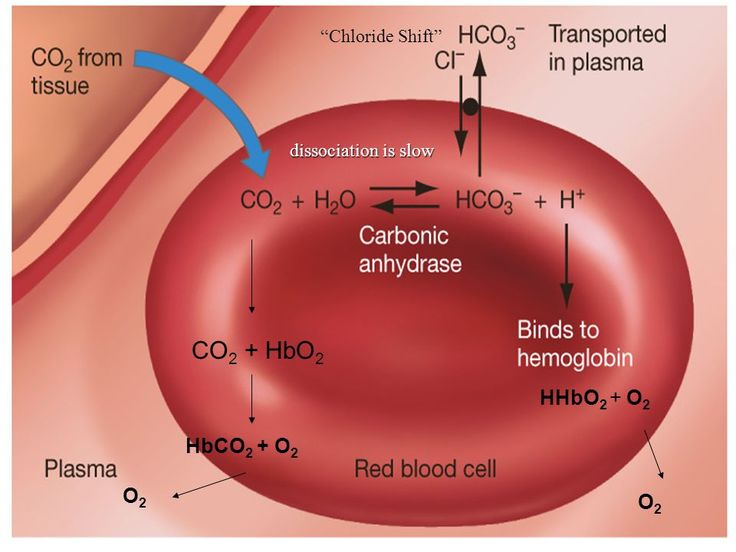
Red Blood Cells (Compensation Mechanisms)
Chloride shift: bicarbonate diffuses out of RBCs during buffering; chloride diffuses in to
maintain electrical neutrality
Isohydric shift: oxygen binding/release from hemoglobin affects H+ release with minimal pH change
Chloride Shift
Bicarbonate diffuses RBC to plasma during buffering, leading chloride to enter RBC to maintain electrical neutrality
Upon CO2 expulsion from the lungs, chloride moves back to plasma and buffers combine with the free H+.
Isohydric Shift
Isohydric = same H+
The process by which blood manages to carry 20 moles of CO2 from peripheral tissues to lungs
Normal arterial blood pH @ 37°C is 7.40 → .05
Normal venous blood pH @ 37°C is 7.37 → .05
Both lungs and kidneys play a major role in maintaining blood pH.
Isohydride Shift: Lungs
Oxygen from the lungs forms oxyhemoglobin in blood. H+ from deoxyhemoglobin in venous blood combines with HCO3- to form carbonic acid, which dissociates into CO2 and H2O. CO2 is exhaled, buffering H+, resulting in minimal pH change.
Isohydride Shift: Kidneys
Kidneys reabsorb bicarbonate (HCO3-) in the proximal convoluted tubule. Aldosterone promotes Na+ reabsorption, exchanging it for excess K+ or H+. Renal cells, rich in carbonic anhydrase, provide a continuous bicarbonate supply.
H+ ions secreted with Na+ may react with phosphate to form phosphoric acid. Glutamate dehydrogenase converts glutamic acid to NH3, which combines with H+ to form NH4+ for excretion. The process restores sodium and bicarbonate in plasma while excreting H+, ammonia, and non-volatile acids.
Base Excess (BE)
Calculated perimeter which describes excess or deficit of base or bicarbonate
+ BE = Increased base
- BE = decreased base
Normal BE 0 ± 2
Decreased base excess is an indicator of metabolic acidosis
Increased base excess is an indicator of metabolic alkalosis
Blood Gas Analysis (ABG)
A test that measures the levels of oxygen, carbon dioxide, and pH in the blood to assess respiratory and metabolic function.
ABG Sample Requirements
Arterial puncture required if PO2 is to be measured (venous blood almost always has PO2 = 40 mmHg)
No tourniquet and no fist-clenching during collection
Use glass syringe and do not pull on plunger; do not use vacutainer
Only heparin (liquid or dry) is acceptable; other anticoagulants alter pH
Protect from air (anaerobic) to prevent equilibration with atmospheric CO2 and O2
Immediately expel any small bubbles
Keep sample submerged in ice/water slush to retard WBC metabolism
pH decreases ~0.08 pH/hr @ 37°C but 1/10th that much @ 0°C
Results are stable for 1-2 hours @ 0°C
Volume of blood for most commercial electrodes is
Reference Ranges: Acid and Base
pH: 7.35-7.45
PCO2: 35-45 mmHg
HCO3-: 22-26 mEq/L
Base Excess (BE): 0 ± 2 mEq/L
PO2: 80-100 mmHg (arterial)
Base Excess (BE)
Calculated parameter describing excess or deficit of base or bicarbonate
+BE = Increased base (metabolic alkalosis
-BE = Decreased base (metabolic acidosis
Normal BE = 0 ± 2 mEq/L
Clinical Assessment: Acid-Base
Identify primary acid-base disorder
Assess degree of compensation
Evaluate underlying cause
Monitor treatment effectiveness