Chemistry Midterm 3 Flashcards
1/105
Earn XP
Description and Tags
Units 8.D-11.C (Quantum Model of the Atom, Periodictiy and Ionic Bonding, Covalent Bonding, Molecular Shape and Bonding Theories, Liquids and Solids)
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
106 Terms
Wave Function
Describes the quantum state of an electron in an atom, specifically finding the probability of the electron at a certain point in space
Shape of s orbital (ℒ = 0)
Spherically symmetric and lack directionality, meaning the probability of finding an electron is uniform on each sphere around the nucleus
Nodes
Points or surfaces where the wave function is zero; number of total nodes is n - 1
Radial Nodes
Spherical surfaces where the wave function is zero; number of radial nodes is n - ℒ - 1
Shape of 2s orbital (ℒ = 0)
Has a sphere with one radial node (the wave function changes from positive to negative)
Angular Nodes
Planar and cone-shaped nodes where the wave function is zero
Shape of p orbital (ℒ = 1)
Dumbbell-shaped; has one angular node in between the two lobes of the dumbbell
Shape of d orbital (ℒ = 2)
Mostly clover-leafed shaped; has two angular nodes
Shape of f orbital (ℒ = 3)
Visually complicated; has three angular nodes
Trend between energy levels (n), orbital size, and energy
As the value of n increases, the orbital size, energy, and radial nodes generally increase
Trend within a shell for orbital energy and size
Within a shell, orbital energy and size increase with ℒ (s —> p —> d —> f)
Trend between number of angular nodes and ℒ
The number of angular nodes in an orbital is equal to ℒ (that’s why it’s called angular momentum quantum number)
Atomic Orbital Energy Diagram
Graphical representation of occupancies and energies of atomic orbitals for each subshell (s, p, d, f)
Orientation of Atomic Orbital Energy Diagrams
Energy is the vertical axis
Orbitals are represented with horizontal lines and labels
Electrons are shown through arrows pointing up (ms = +1/2) and down (ms = -1/2)
Aufbau Principle
Electrons fill the lowest-energy orbitals (ground state) first before occupying higher-energy orbitals
Hund’s Rule
Every orbital within a subshell is singely occupied with one electron before any orbital is double-occupied (single-occupied orbitals must have the same spin)
Electron Configuration
Lists the arrangement of electrons within each orbital for the subshells. For ground state electron configurations, it is listed in their lowest possible energy states
Ex. For a neutral sulfur atom: 1s22s22p63s23p4
Degenerate
Orbitals within a subshell
Highest-energy occupied subshell for s and p-block elements
Period number
Highest-energy occupied subshell for d-block elements
Period number - 1
Highest-energy occupied subshell for f-block elements
Period number - 2
Abbreviated Electron Configurations
Shorthand way of writing electron configurations using noble gases
Ex. For a Ca atom, it would be [Ar]4s2
Valence Shell
Highest-energy occupied shell
Valence Electrons
Electrons that are within the highest-energy occupied shell involved in bonding and electron-transfer processes
Ex. For a F atom (1s22s22p5), there are seven valence electrons in n=2 shell (2s22p5)
Core Shell
Lower-energy occupied shells
Core Electrons
Electrons within lower-energy occupied shells that shield valence electrons from full positive charge of nucleus; aren’t involved in chemical reactions usually
Ex. For a O atom ([He]2s22p4), the core electrons are within the [He}
Subshells relating to Cations for Main Group (s and p-block) Elements
Main group (s and p-block) elements in groups 1,2, and 13 form cations to become more stable, specifically to the closest noble gas
Subshells relating to Cations for Transition (d-block) Elements
Transition (d-block) elements form cations by removing electrons from the highest principal energy level (n) first
Ex. Fe has [Ar]4s23d6, but when it’s Fe2+, it loses the 4s2 electrons to become [Ar]3d6
Subshells relating to Anions for Main Group (s and p-block) Elements
Main group (s and p-block) elements in groups 14-17 are nonmetals that want to complete their valence shell and become more stable by configuring to nearest noble gas
Size of atoms are dictated by-
Electronic structure of the atom or ion
Interactions between the positive nucleus and the negative electrons
Interactions between the positive nucleus and the negative electrons follow-
Electrostatic Principles
Electrostatic Principles
Oppositely-charged particles attract each other
Like-charged particles repel each other
As charges increase, so does the forces of attraction or repulsion
As two charged bodies get closer to each other, so does the forces of attraction or repulsion
Effective Nuclear Charge (Zeff)
The net positive charge from the nucleus that a valence electron experiences
Zeff for Valence Electrons
They are lower than the actual nuclear charge (Z), thus Zeff < Z due to the shielding effect by core electrons and increased distance
Formula for Zeff
Zeff = Z - S
Z: Nuclear Charge (number of protons)
S: Shielding Constant
Slater’s Rules
Helps determine the shielding constant (S) for a valence electron in an atom using only the number of core and valence electrons
Formula for Shielding Constant (S)
S = 0.85(Ncore) + 0.35(Nval - 1)
Ncore = number of core electrons
Nval = number of valence electrons
Atomic Radius
Measure of the size of the atom based on the distance between the nucleus and the outermost electron shell
Atomic Radius Trends
Increases down a group
Principal energy levels (n) are added, thus this expands the atomic size
Decreases from left to right across a period
As the number of protons increase, they pull in the electrons more tightly, thus force of attraction increases
Applies to cations and anions as well
Atomic Radius for Cations
All cations have smaller radii than their corresponding neutral atoms
With less electrons, this lowers the shielding effect, thus the remaining electrons are pulled in more tightly towards the nucleus
Atomic Radius for Anions
All anions have larger radii than their corresponding neutral atoms
The addition of electrons increases electron-electron repulsion and shielding, thus the atom expands to so there is more distance between the electrons
Ionization Energy
Energy required to remove an electron from the gaseous atom to produce a gaseous cation and a free electron
Ionization Energy Trends
Decreases going down a group
The outermost electrons are farther from the nucleus, thus it requires less energy to remove an electron due to less forces of attraction
Increases going left to right across a period
As the number of protons increase, this leads to the electrons being pulled more tightly, thus it’s harder to remove them
Exceptions to Ionization Energy
Outermost valence electron is in a subshell by itself
Elements containing one set of paired electrons in the valence p subshell (Group 15 vs. Group 16)
Higher Ionization Energies:
Involves the removal of a second electron, third electron, etc.
Zeff increases with each electron lost, thus the attraction between the electrons and the nucleus become stronger, thus that’s the reason for higher ionization energy as electrons are removed
Electron Affinity
Energy change when an electron is added to a gaseous neutral atom to create a gaseous anion
Electron Affinity Values
Positive value means an increase in energy needed, thus an endothermic process
Negative value means an decrease in energy needed, thus an exothermic process
Electron Affinity Trends
Becomes less exothermic (less negative) moving down a group due to less energy released when an electron is added farther away from the nucleus
Becomes more negative left to right across a period (ignoring noble gases) due to stronger pull from nucleus for incoming electron, thus more energy is released when added
Electrostatic Attraction
Forces of attraction between oppositely-charged species
Ionic Bond
The electrostatic attraction between oppositely-charged ions
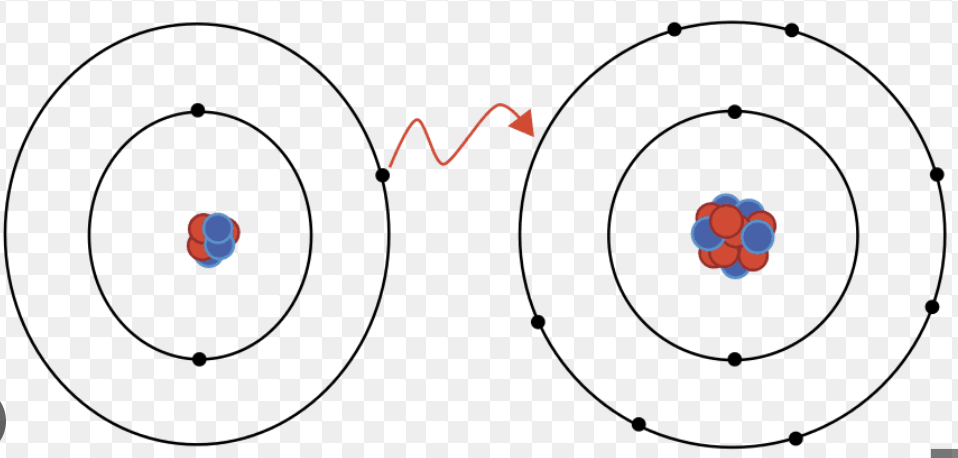
Ionic Bond Characteristics
Metal donates electron to the non-metal that accepts it to achieve the electronic structure of the nearest noble gas
Compounds contain an infinite 3D lattice of cations and anions (formula unit)
Lattice Energy (△HL)
The energy released when gas-phase ions combine to form 1 mole of a solid ionic compound (can be reversed where a solid ionic compound become gas-phase ions but it’s endothermic)
Lattice Energy Trends
A decrease moving down a group because as atomic size increases, there’s a weaker electrostatic attraction between the positive and negative ions
An increase from left to right across a period due to ionic charge increasing, which creates a stronger electrostatic attraction with nucleus and the electrons
Trends are based on Coulomb’s Law
Coulomb’s Law
As the charge increases, the forces of attraction increases (direct relationship)- relates to period trend
As the distance between the center of two ions increase, the forces of attraction decreases (inverse relationship)- relates to group trend
Born-Haber Cycle
Since lattice energies can’t be directly measured, this cycle uses Hess’s Law, ionization energy, electron affinity, and the energy of other processes to calculate △HL
Covalent Bonds
The sharing of electrons between atoms, generally between two nonmetals
Octet Rule
Achieving a stable noble gas configuration (low-energy state) with eight total valence electrons
Why Eight Electrons?
For p-block elements, this correpsonds to a full atomic valence shell (ns2np2) which hold eight electrons
Lewis Structure
Diagram of a molecule or polyatomic ion that shows the shared pairs or bonds through straight lines and the unshared or lone pairs through dots
Total number of electrons in a Lewis structure must equal the total number of valence electrons of the atoms in the molecule
Number of Bonds
Single Bond (weakest)
Holds 2 electrons
Double Bond
Holds 4 electrons
Triple Bond (strongest)
Holds 6 electrons
Steps for Drawing Lewis Structures
G
g
g
g
g
g
g
Formal Charges
Based on the count of valence electrons to obtain a theoretical charge
Formal Charge Formula
(# of valence electrons) - (# of non-bonding electrons) - (1/2 of bonding electrons)
Electronegativity
Measure of capacity or ability of an atom to attract shared electrons in a covalent bond
Electronegativity Trends
Increases left to right across a period due to effective nuclear charge increasing, thus more protons being added to the same energy level brings the electron cloud closer for better attraction
Decreases going down a group since electrons get further away from the nucleus and the core electrons shielding the valence electrons to weaken the nucleus pull
Characteristics of Highly-Electronegative Elements
Have greater electron density, leading to more unequal sharing of electrons (more polar)
Form fewer bonds due to preference of creating one strong bond rather than sharing electrons in multiple bonds
Placed on periphery of Lewis structure to reduce electron-electron repulsion (which destabilizes the atom)
Correlation between Electronegativity and Zeff, Ionization Energy, and Electron Affinity
High Electronegativity means high Zeff, high ionization energy, highly negative electron affinity (except for exceptions)
Mulliken Scale
1/2(IE + EA)
The bigger the values, the tighter the atom holds electrons
Pauling Scale
The difference between X-X and X-Y (stronger due to polarization) bond energies
Shown through electronegativty chart
Exceptions to Octet Rule
Duet Rule: Hydrogen forms one bond (1s2)
Incomplete Octet: Group 13 elements only forms three bonds to remain neutral. Be in Group 2 only forms two bonds.
Expanded Octet: elements in Period 3 and above can expand their valence shell
Resonance Structures
Multiple, equivalent Lewis structures that differ only in the placement of multiple bonds and lone pairs (nuclei and single bonds don’t move)
Resonance Hybrid
Weighted average of all resonance forms that’s closest to the molecule’s “true” structure
Ranking of Contributions Towards Resonance Hybrid
Fewer and small formal charges represent stability
Avoid like charges on adjacent atoms and maintain separation
Negative formal charge should be on most electronegative atom
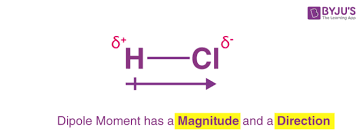
Dipole
The creation of a partial positive charge and a partial negative charge
Polar Covalent Bonds
Unequal sharing of electrons that creates substantial dipoles
Dipole Moment (𝜇)
Measurable quantity of separation of positive and negative charges to indicate overall polarity
Dipole Moment Formula
𝜇 = qr
q = magnitude of charge
r = distance between charges
Units are Debye (D) - 3.34 × 10-30 C x m (C is charge unit Coulomb, m is meters)
Represented as a dipole vector
Bond Enthalpy (Bond Dissociation Enthalpy)
Measure of strength of covalent bonds through the enthalpy change of 1 mole of bonds breaking homolytically in gaseous molecules
Homolytically
When a chemical bond breaks into two uncharged atoms or radicals equally
Bond Enthalpy Formula
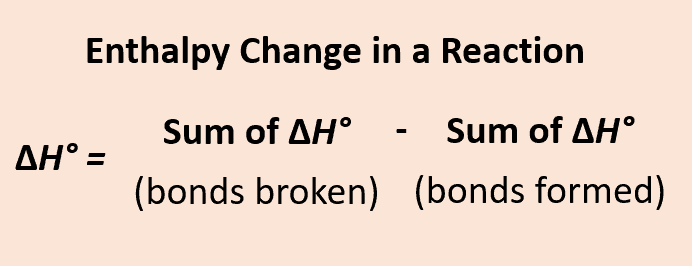
Bond Breaking
Endothermic- energy is added to break bonds
Bond Forming
Exothermic- energy is released as bonds form
Trend between Bond Length and Bond Enthalpy
Inverse relationship
As bond length decreases, the bond enthalpy increases since the electrons are closer, resulting in more energy necessary to break bonds
Bond Order (Strength)
Single Bonds < Double Bonds < Triple Bonds
Why isn’t bond order and bond length linearly correlated?
Inversely proportional with a curved line because the decrease in length is not a constant amount for each increase in bond order
Exothermic Energy Diagram
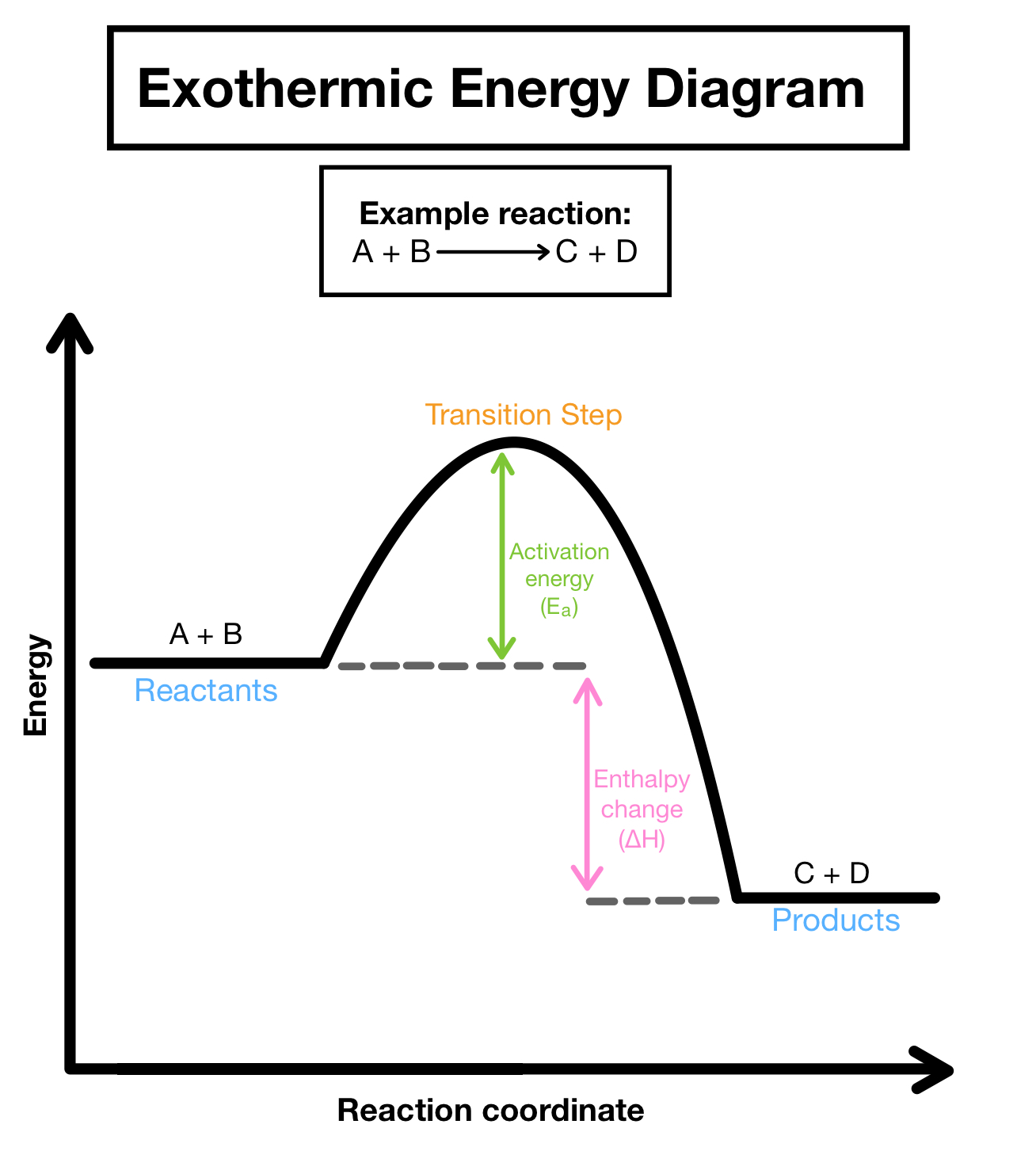
Endothermic Energy Diagram
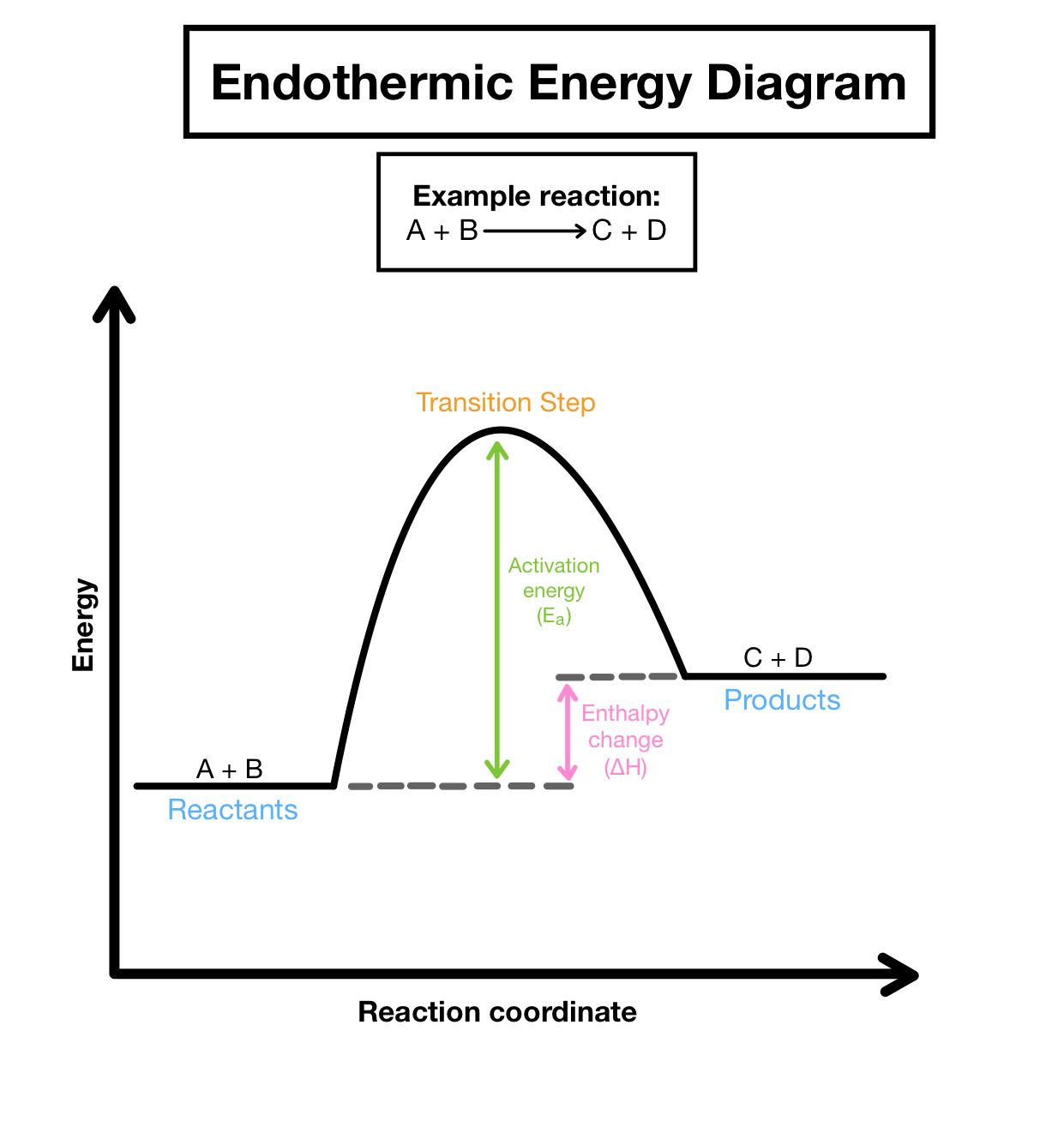
Valence Shell Electron Pair Repulsion (VSEPR):
Model allowing predictions for the shape of the molecule based on number of electron domains surrounding the central atom
Electron Domains
Regions of electron density, including lone pairs, single unshared electrons, bonds (single, double, triple)
Electron Geometry
Arrangement of electron domains around the central atom. Doesn’t matter if it includes the bonding and non-bonding electrons
Molecular Geometry
3D-shape arrangement of atoms around the central atom.
Steric Number
Sum of number of atoms bonded to the central atom plus the number of lone pairs of electrons on that central atom
Linear Electron Geometry
Steric Number- 2
180o
Trigonal Planar Electron Geometry
Steric Number- 3
120o
Tetrahedral Electron Geometry
Steric Number- 4
109.5o
Trigonal Bipyramidal Electron Geometry
Steric Number- 5
Angles depend on orientation
Axial- along the vertical axis
Equitorial- along the equitorial plane
Lone pairs occupy equitorial positions
Octahedral Electron Geometry
Steric Number- 6
Angles depend on orientation
Molecular Dipole
Molecules with uneven electron distribution (polar); have measureable dipole moments
Polar Molecule
Molecules with asymmetrically-arranged polar bonds around the central atom; the bond dipoles will create a molecular dipole
Non-polar Molecule
When polar bonds are arranged symmetrically around the centrl atom to cancel the bond dipoles