A-Level Chemistry Shapes of molecules
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
4 bonding pairs, no lone pairs
Tetrahedral

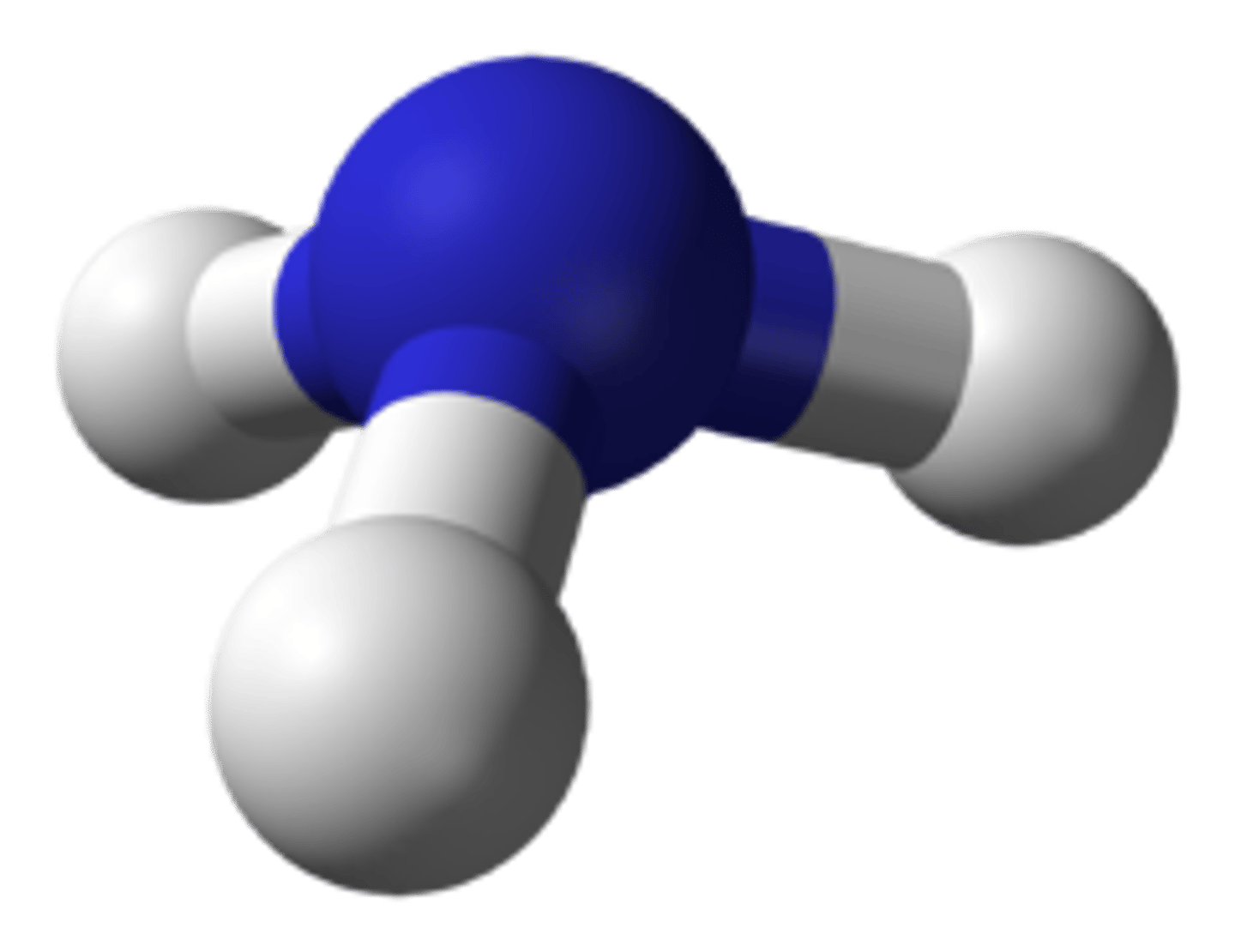
3 bonding pairs, 1 lone pair
Trigonal Pyramidal
2 bonding pairs, 2 lone pairs
Bent planar
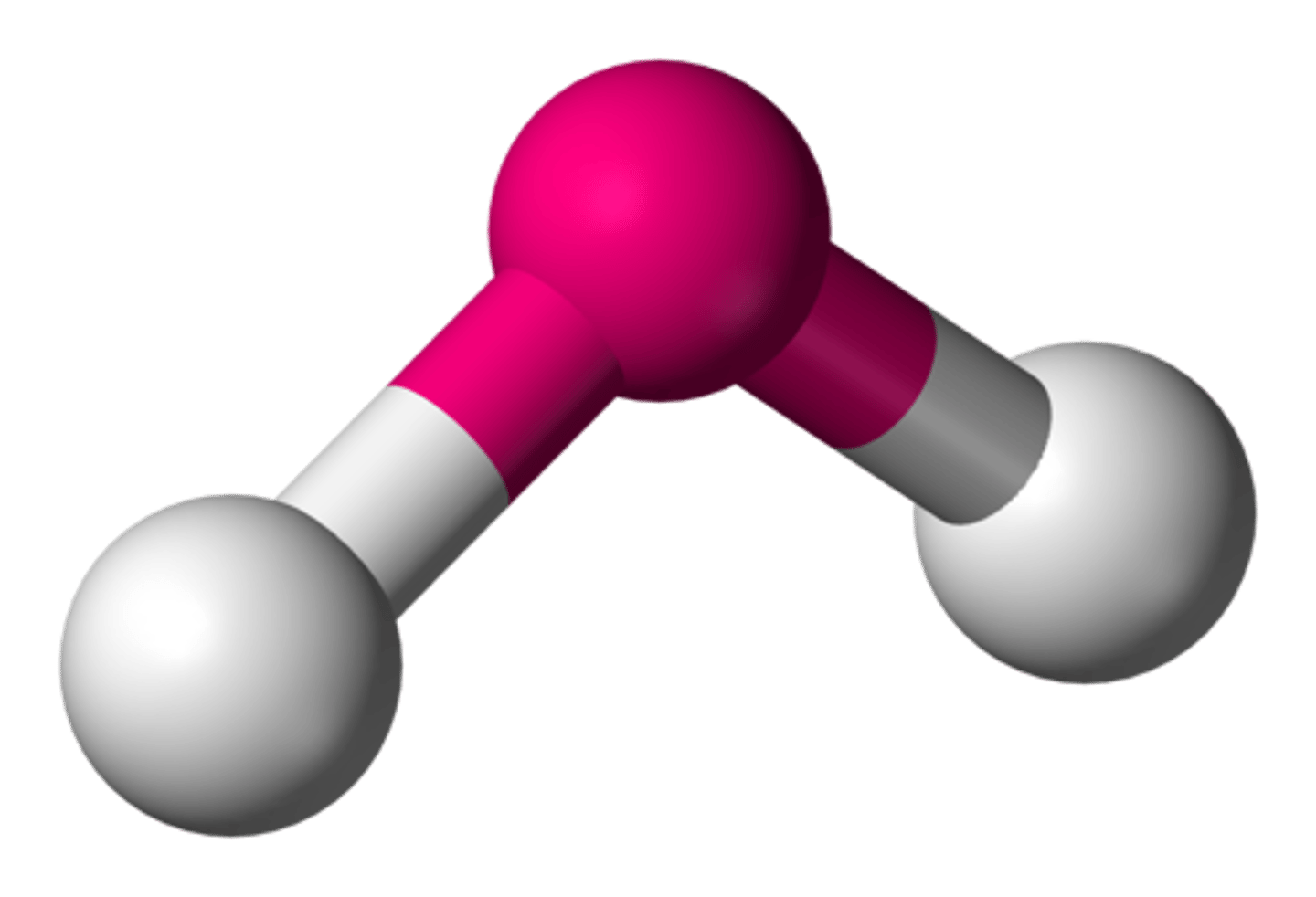
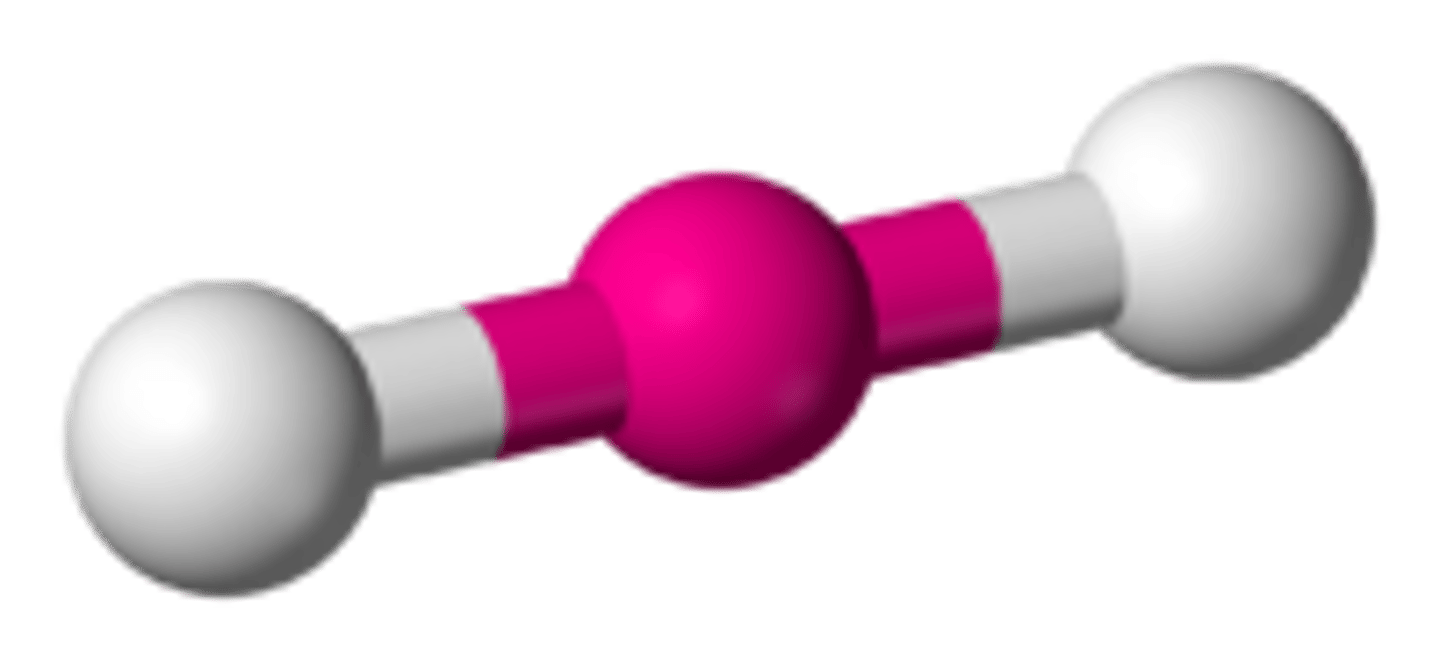
2 bonding pairs, no lone pairs
Linear
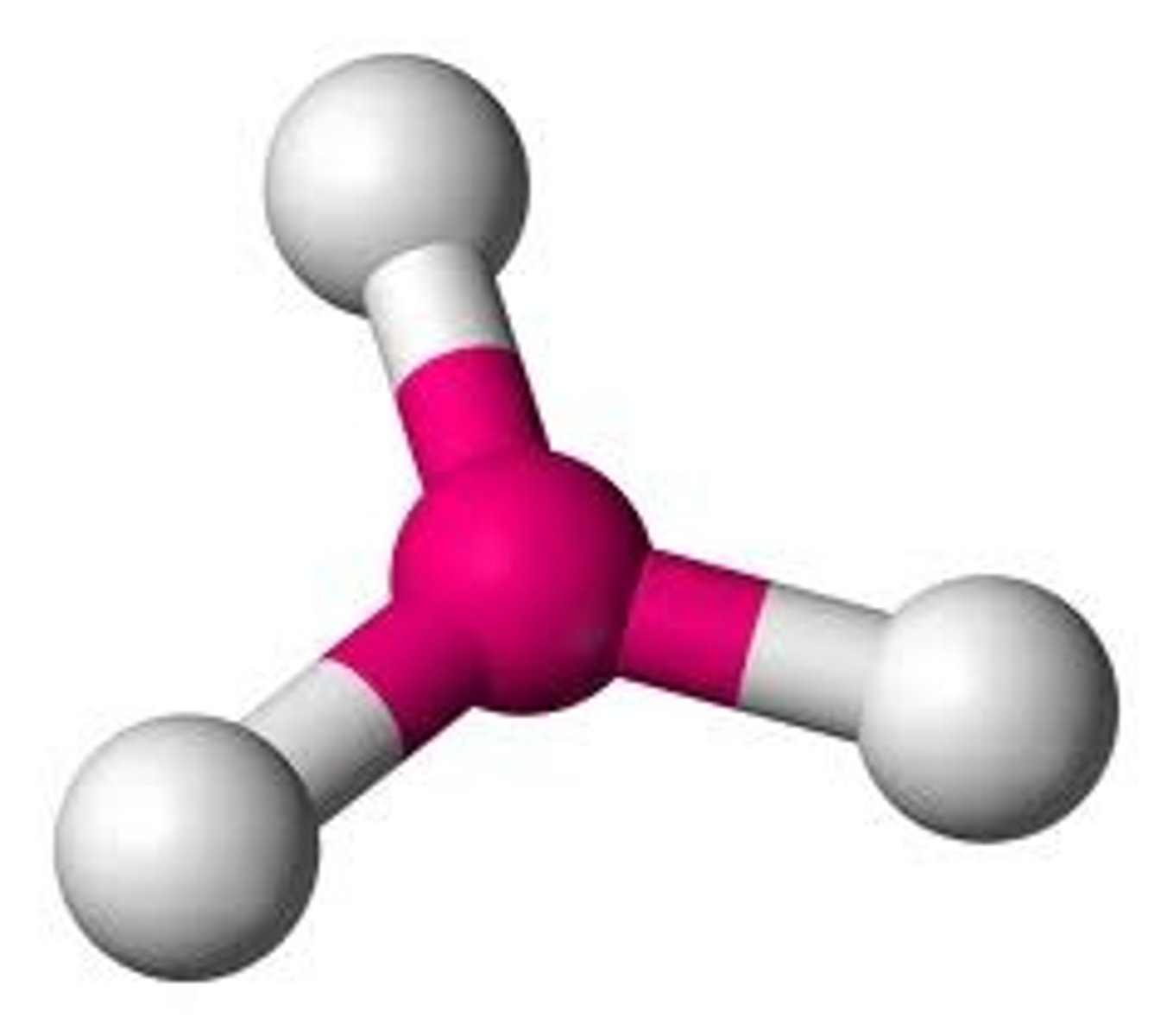
3 bonding pairs, no lone pairs
Trigonal planar
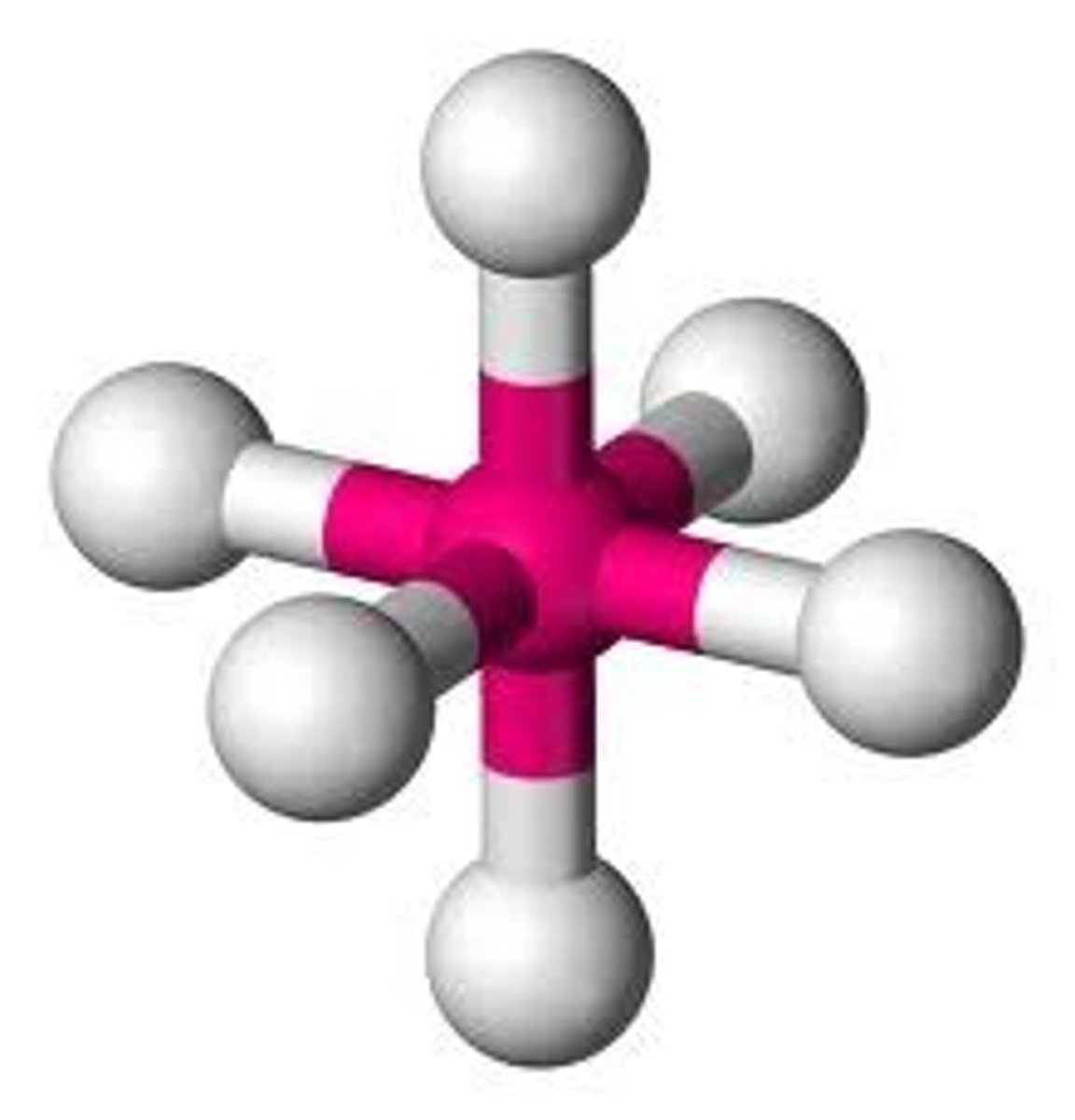
5 bonding pairs, no lone pairs
Trigonal bipyramidal
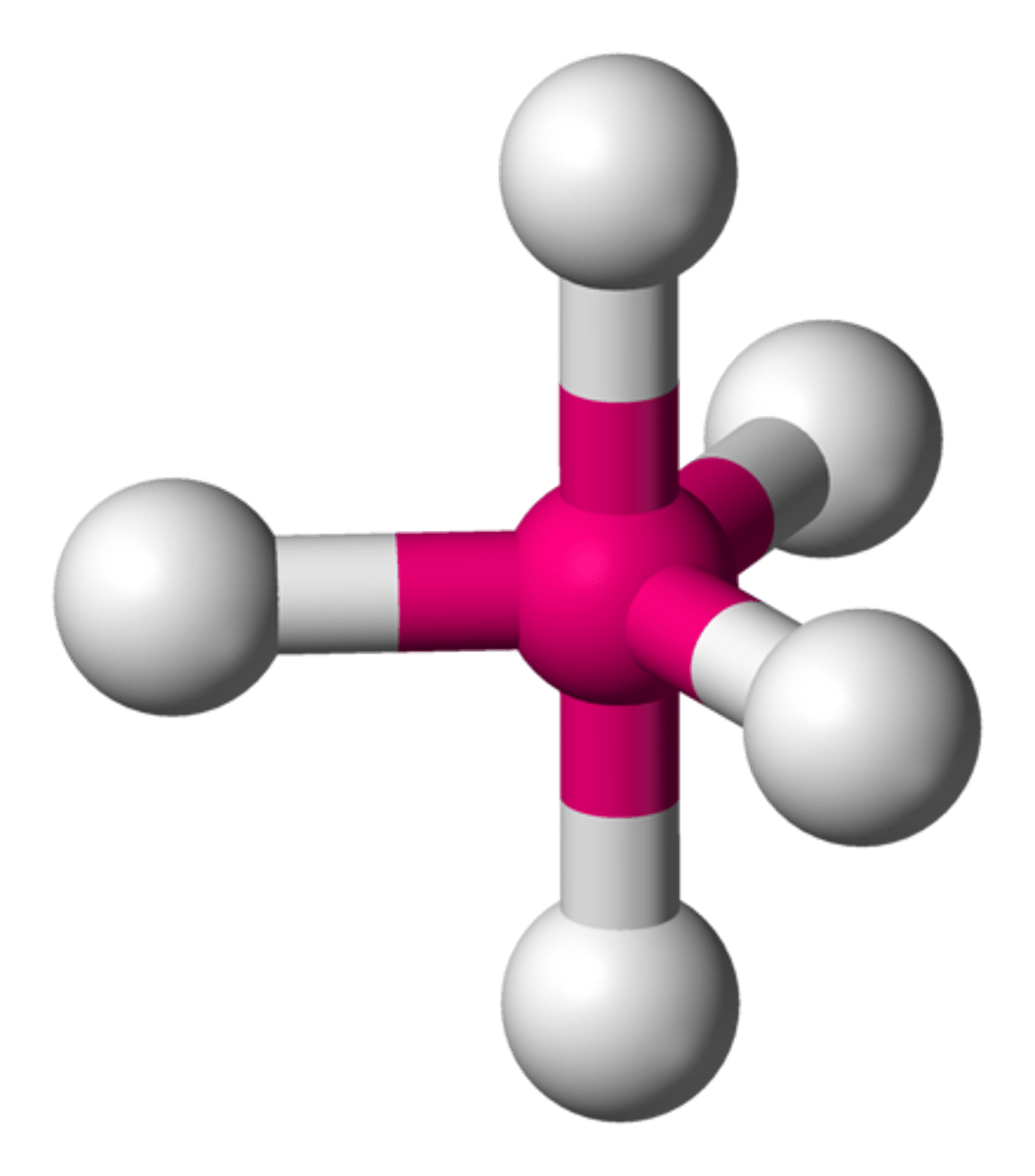
6 bonding pairs, no lone pairs
Octahedral
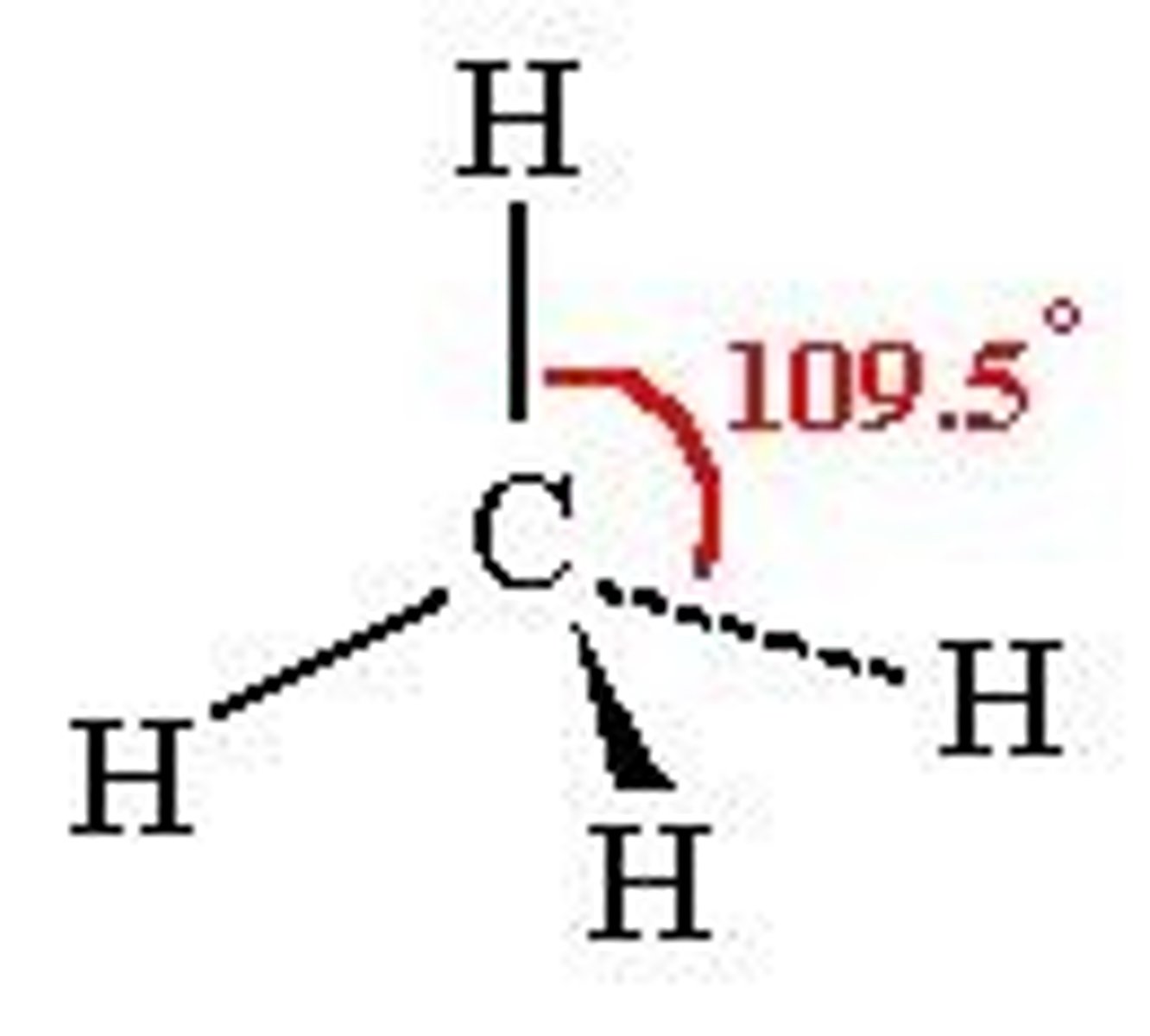
Tetrahedral bond angle
109.5°
Trigonal pyramidal bond angle
Around 107°
Bent planar bond angle
Around 104.5°
Linear bond angle
180°
Trigonal planar bond angle
120°
Trigonal bipyramidal bond angles
90° and 120°
Octahedral bond angles
90°
Electron pair repulsion theory
A theory which explains the shapes of simple molecules by assuming that pairs of electrons around a central atom repel each other and thus take up positions as far away as possible from each other in space.
3 bonding pairs, 2 lone pairs
T-shaped
4 bonding pairs, 2 lone pairs
Square planar - lone pairs go in axial positions.
2 bonding pairs, 3 lone pairs
Lone pairs go in equatorial position so linear.
