Bio Final Exam-Squarecap
1/221
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
222 Terms
When sodium with one electron and chlorine with 7 electrons in their valence shells respectively interact, they will form
ionic bonds
What type of bond will break when you blow dry hair?
H-bonds
**hydrogen bonds can break with both water and heat
Which of the following is the correct ranking of three bonds and interactions in order from highest to lowest in terms of their bond strength?
1. Disulfide (-S-S-) bond between two cysteines
2. Hydrophobic interactions between two leucines
3. H-bonding in water
1,3,2
1-covalent bond
2-hydrophobic bond
3-hydrogen bond
**Ionic
Biologists say covalent bonds are stronger than ionic bonds and chemists say ionic bonds are stronger than the covalent bonds.
Both are correct but what makes the difference?
Covalent bonds are more likely to happen in aqueous reactions making them stronger in biological situations compared to the view of chemists.
Soap can be used to remove both grease (non polar) and salt (polar). What property makes soap so versatile?
soap is amphipathic
**both hydrophobic and hydrophillic
What is the chemical that can be found in your kitchen that will break down the bubbles and eliminate foaming?
Salt
*it stops the formation and merging of bubbles
How does spreading salt on the roads reduce ice formation?
Salt is an ionic compound. When it is added to the roads it lowers the freezing point of water making it harder for the water to freeze over the roads.
Which of the following is the most important property of water to support life?
Reactant and Medium
**Water can be both a reactant and medium for chemical reactions to take place
Even though H-bonding occurs in ammonia and ethanol, they cannot be used to replace water in living cells because _______
water is an essential property in life and the body cannot live without it
Organic compounds are _________
carbon based compounds, generally derived from living organisms
which functional groups will make a good buffer?
Carboxyl, amine, phosphate
**weak acids or weak bases make best buffers
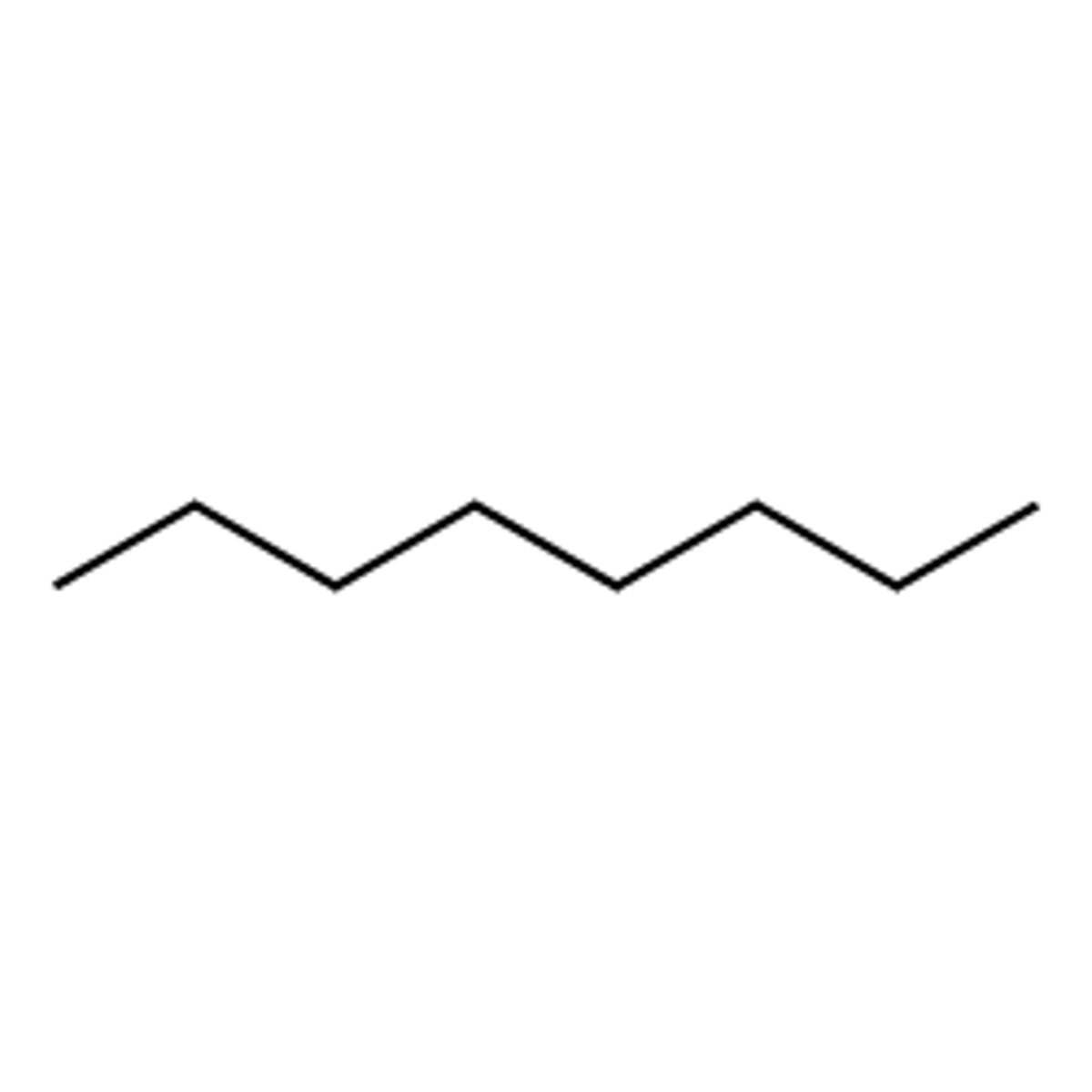
What is the nature of the molecule?
highly non-polar and insoluble in water
**octane-8 carbon long hydrocarbon
molecules containing hydroxyl (-OH) group are referred to as an/a _________
alcohol
The molecule shown above has the properties of a/an ____________
both an acid and a base
**molecule has both amine and acid group, it has properties of both acid and base.
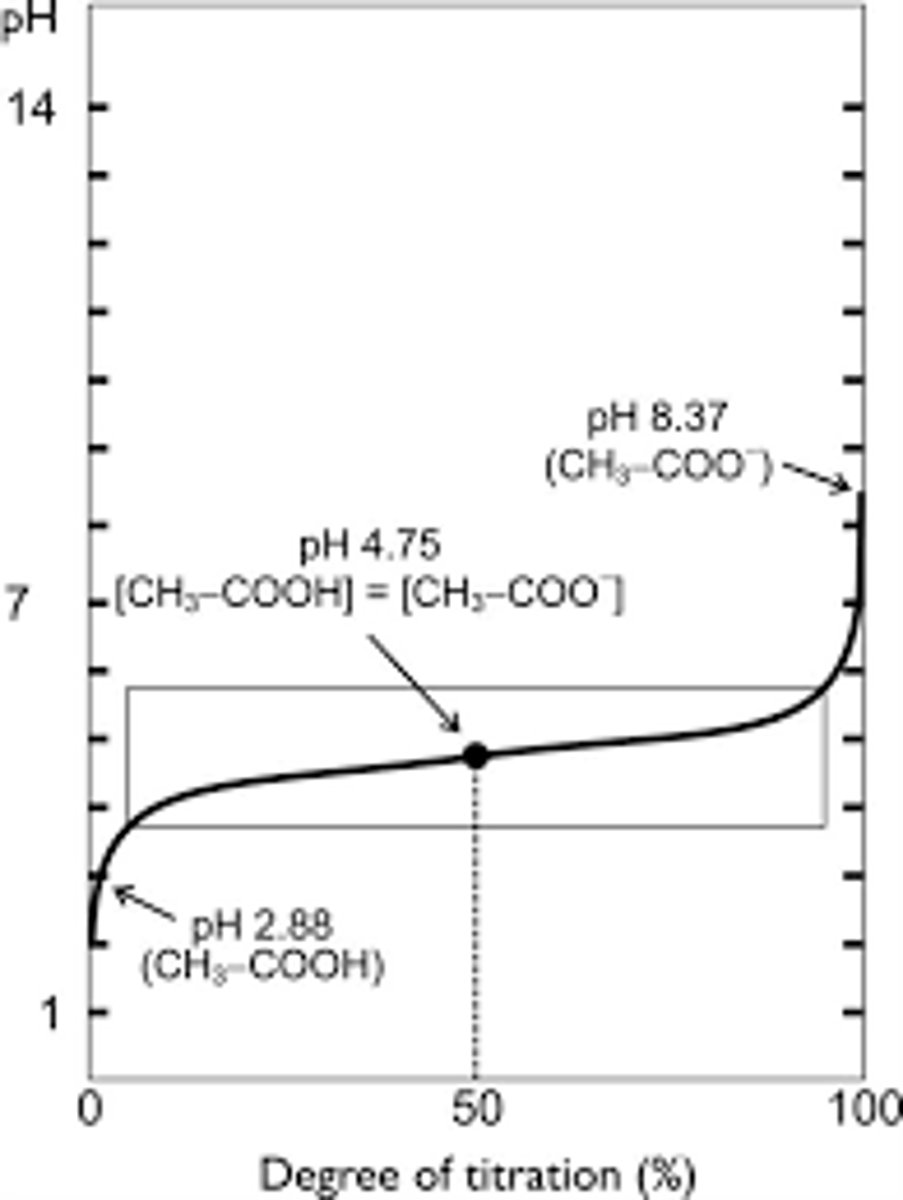
Based on the above graph, all the acetic acid will become conjugate base at the pH _______
8.37
**because it is becoming a weak base outside of the buffer range
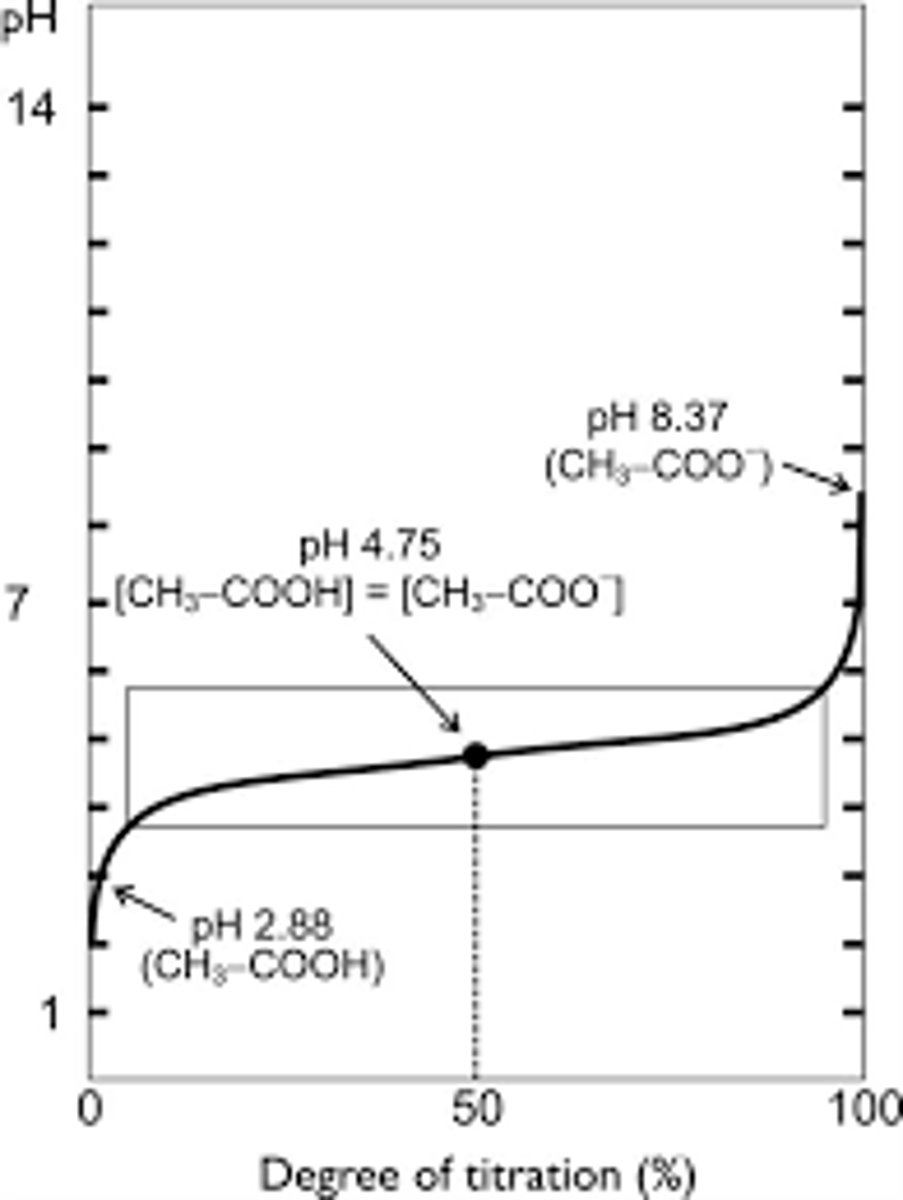
Based on the above graph, the pK for acetic acid is _________
4.75
*pK is the ratio of an acid form to the base form is one. at this pH, the concentration of CH3-COOH is equal to the concentration of CH3-COO-.
If you want to design a drug that will easily enter the non-polar biological membrane, which of the following functional group would be the best to add to such molecule?
Methyl group
**Methyl is non-polar and the more non-polar molecules there are, the easier it can pass through the non-polar membrane.
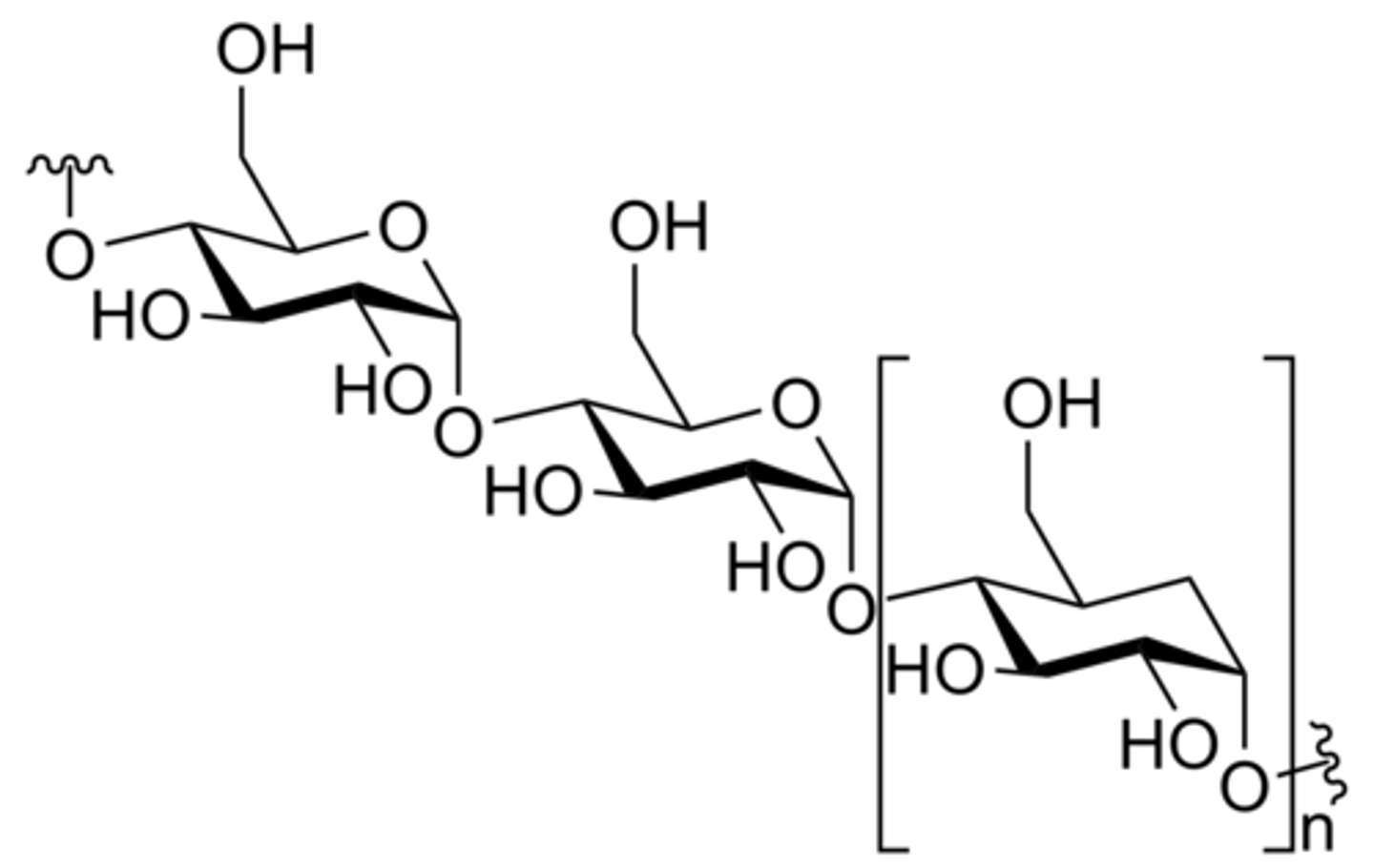
Consider a starch consisting of 25 glucose molecules. the complete hydrolysis of this polysaccharide would result in the production of ___________
25 glucose molecules
**the starch is made up of 25 glucose molecules, so when it is broken down into monomers, it will be 25 glucose molecules remaining.
simple sugars mostly contain C,H and O in the ratio of_____________
1:2:1
***bc glucose molecule is C6H12O6, 6:12:6 = 1:2:1
The Molecule of glucose (shown below) when dissolved in water will behave as a/an___________
alcohol
***
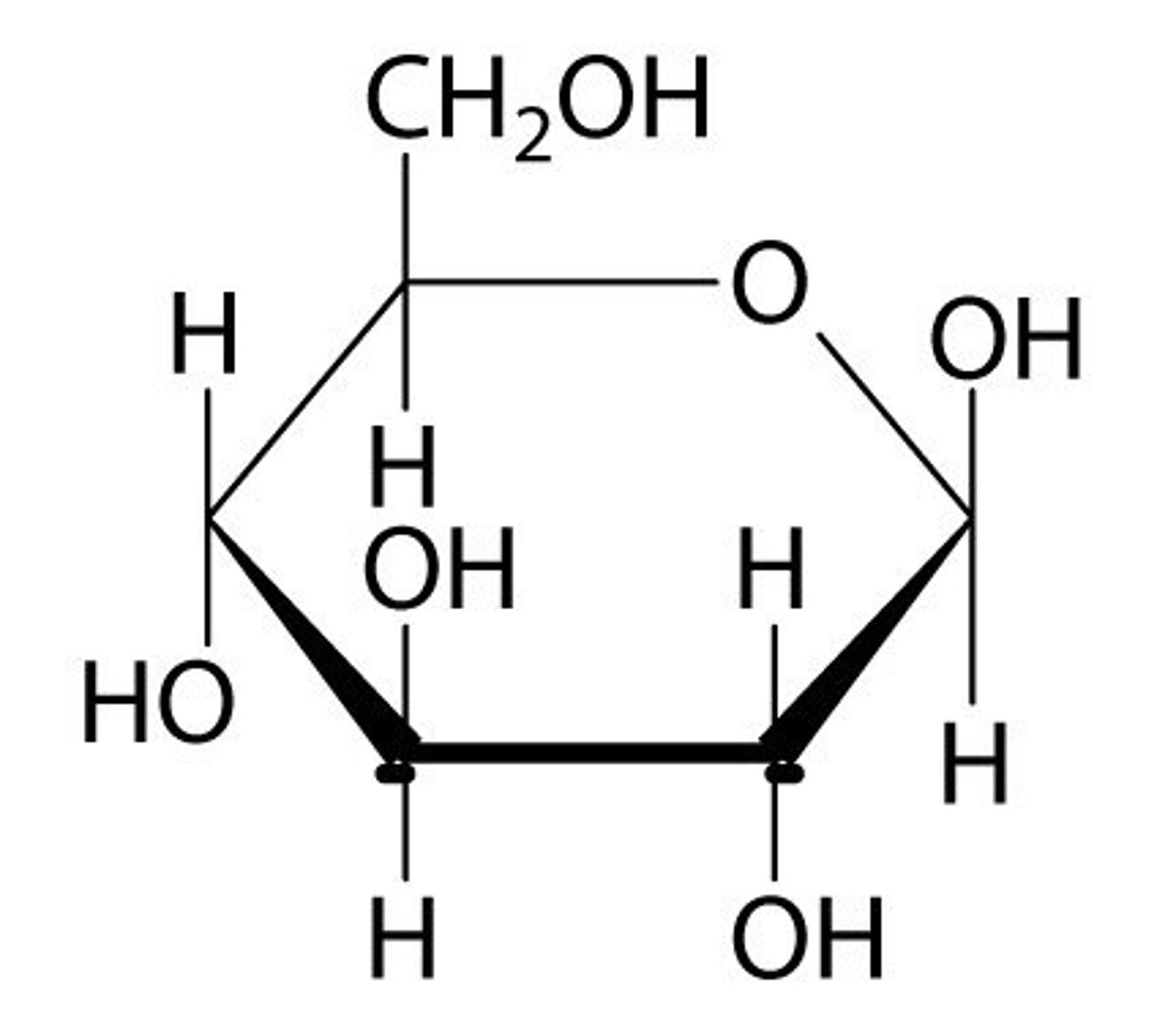
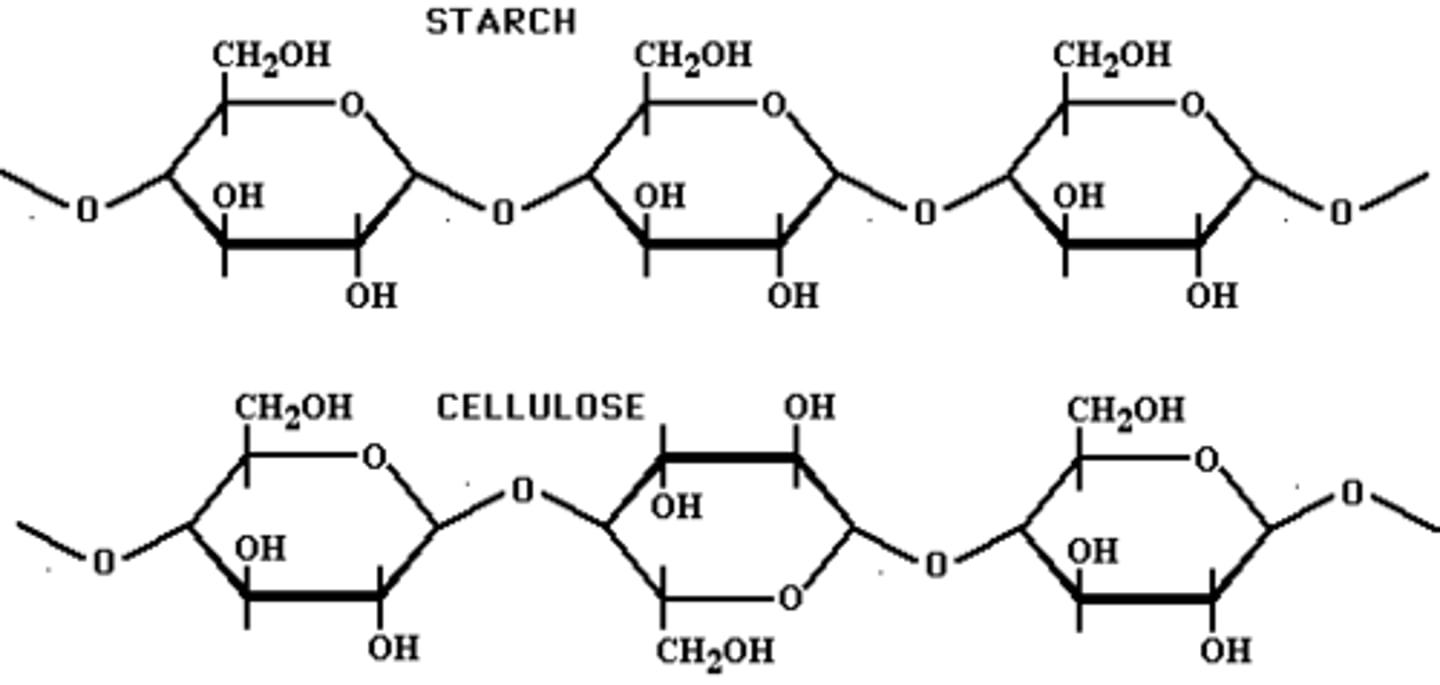
We are able to digest starch but not cellulose bc we _____________
starch is a storage polysaccharide while cellulose is a structure polysaccharide, and cellulose is used in plant cell walls and since humans don't have cell walls they won't need the cellulose
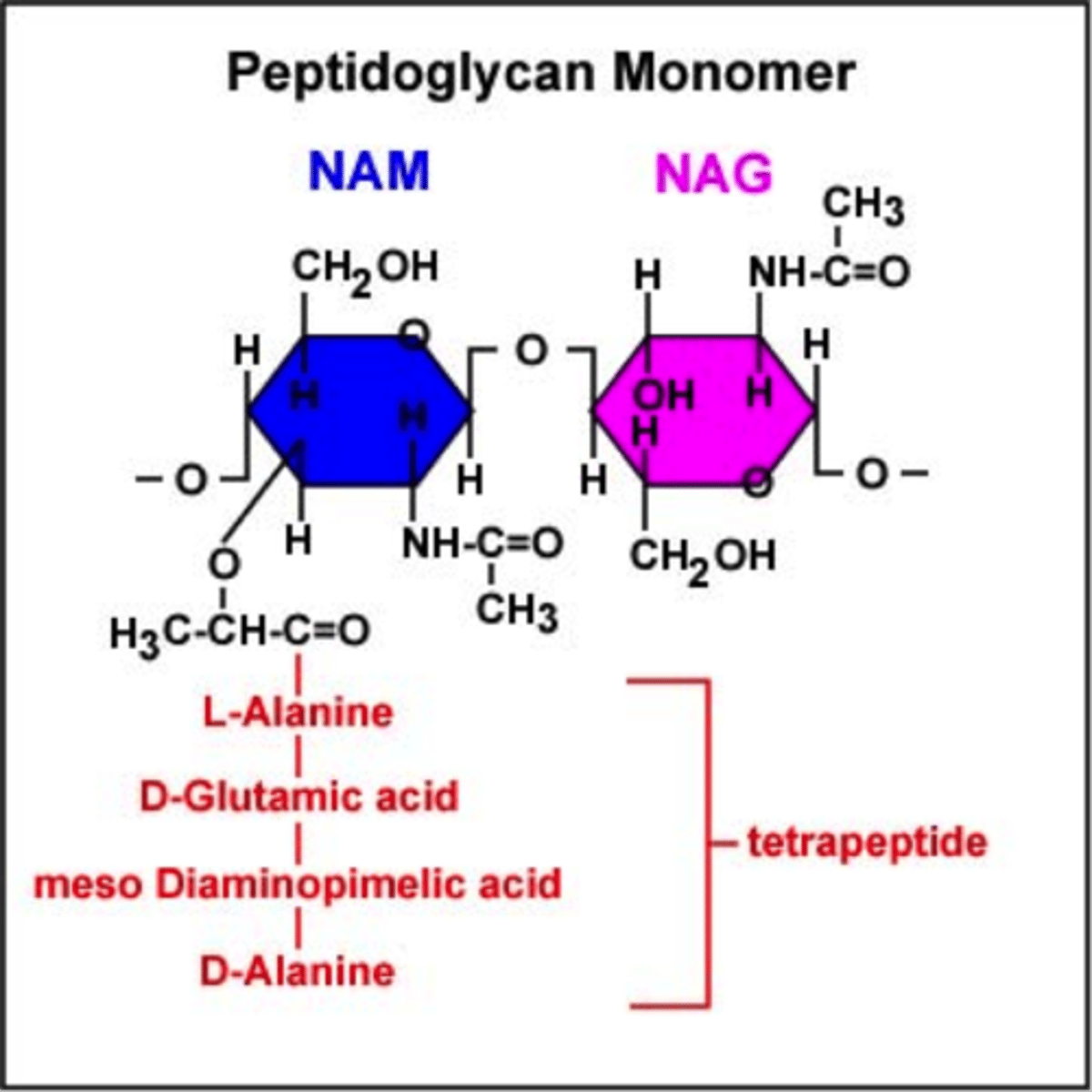
You are trying to attach a radioactive label to the cell wall polysaccharide in bacteria by growing the bacteria in a radioactive medium. Which of the following element will not label the polysaccharide NAM-NAG (N-acetyl muramic acid-N-acetyl glucosamine) in bacterial cell wall?
Phosphorus
**bc we are talking about sugars and they don't contain phosphorus. Only DNA/RNA have phosphorus
Which of the following molecule will have the molecular formula C12H22O11?
A disaccharide
***bc it is about double the regular formula of glucose, but think of n-1 for H and O have a couple less
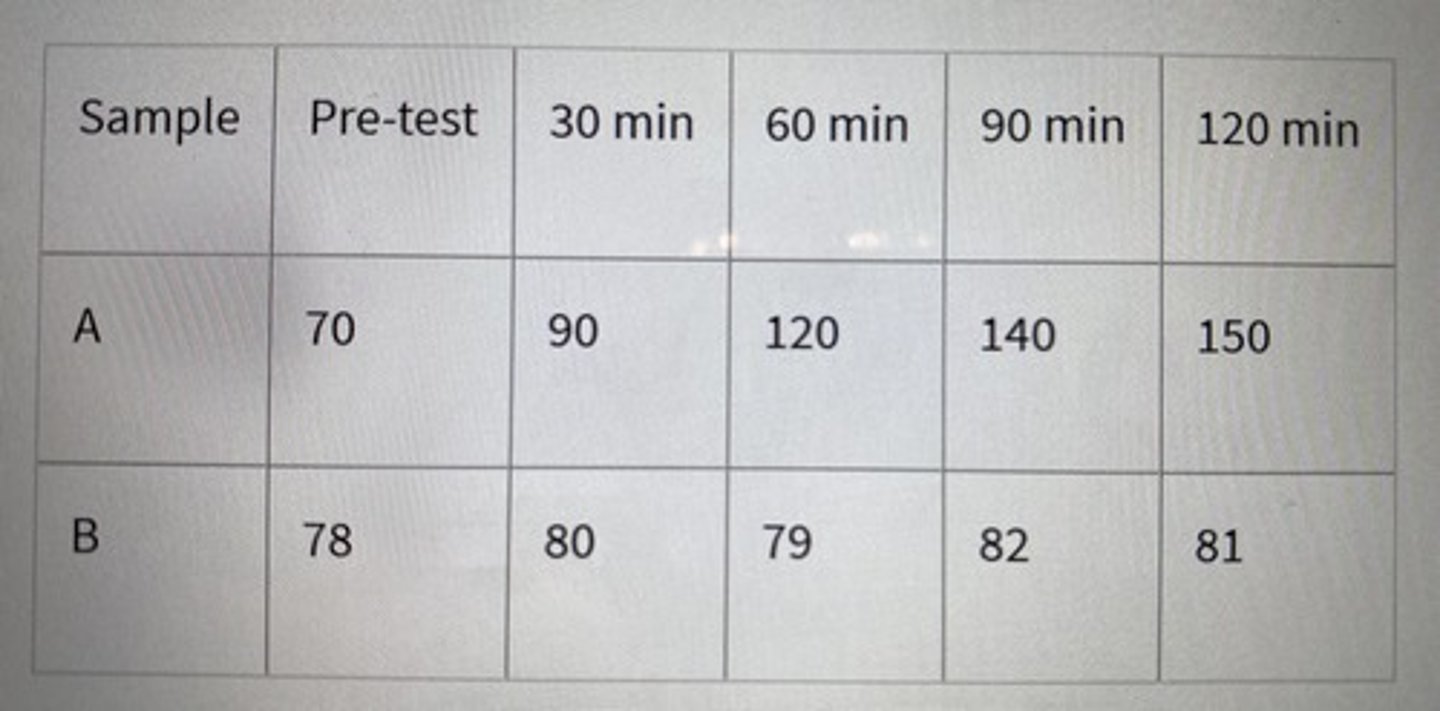
John Doe visits a doctor complaining of stomachache after drinking milk every time. Doctor asks John to fast for 8 hours and then drink regular milk to tests his blood glucose level (mg/dL) every 30 minutes. Shown below are data from two blood samples taken from John and another control patient undergoing similar diagnosis but does not have lactose intolerance. Predict which sample belongs to John Doe if he had lactose intolerance and which is the control blood sample? Note: Lactose intolerance is due to the inability to break down lactose to glucose and galactose.
Blood glucose level (mg/dL)
Which sample belongs to John Doe and why?
Sample B bc he has lactose intolerance and hence his glucose level stays the same.
Which of the following bonds would strengthen storage polysaccharide in dry grains in addition to covalent bonds?
H-bonds
***
Lipids in general are_________
molecules used to aid in storage, protection/ cushioning of vital organs, insulation, and a major component of the lipid bi-layer
Which of the following lipids are generally considered as neutral or non-polar lipids? Select all that apply.
Fats, steroids, carotenoids, and waxes.
***I know waxes are non polar, like when you wax a car
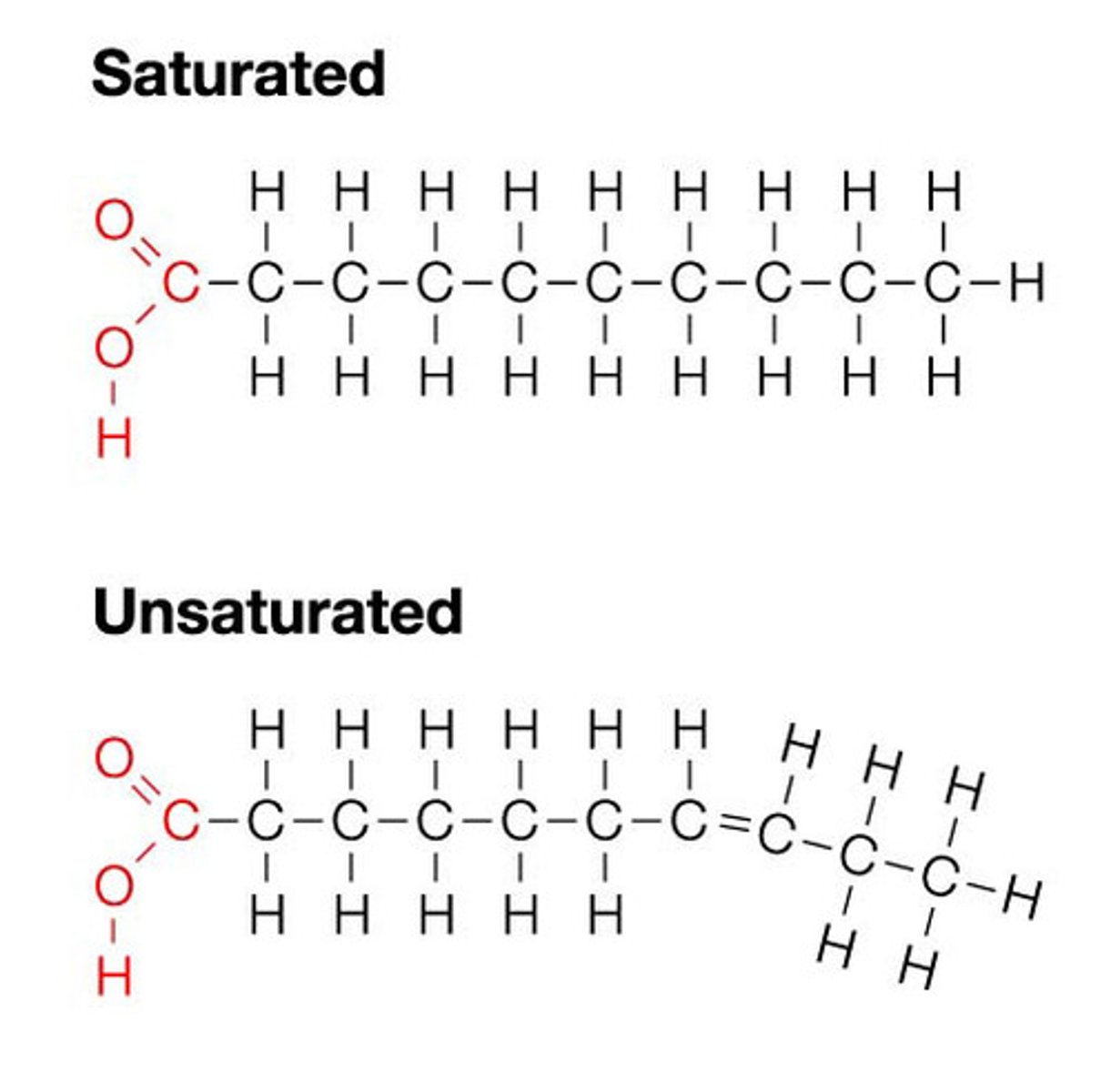
As the saturation of fatty acid increases due to the enzyme saturase activity, the melting temperature (Tm) of such modified fatty acid will ________________
increase
***bc saturated fats are solids at room temperature
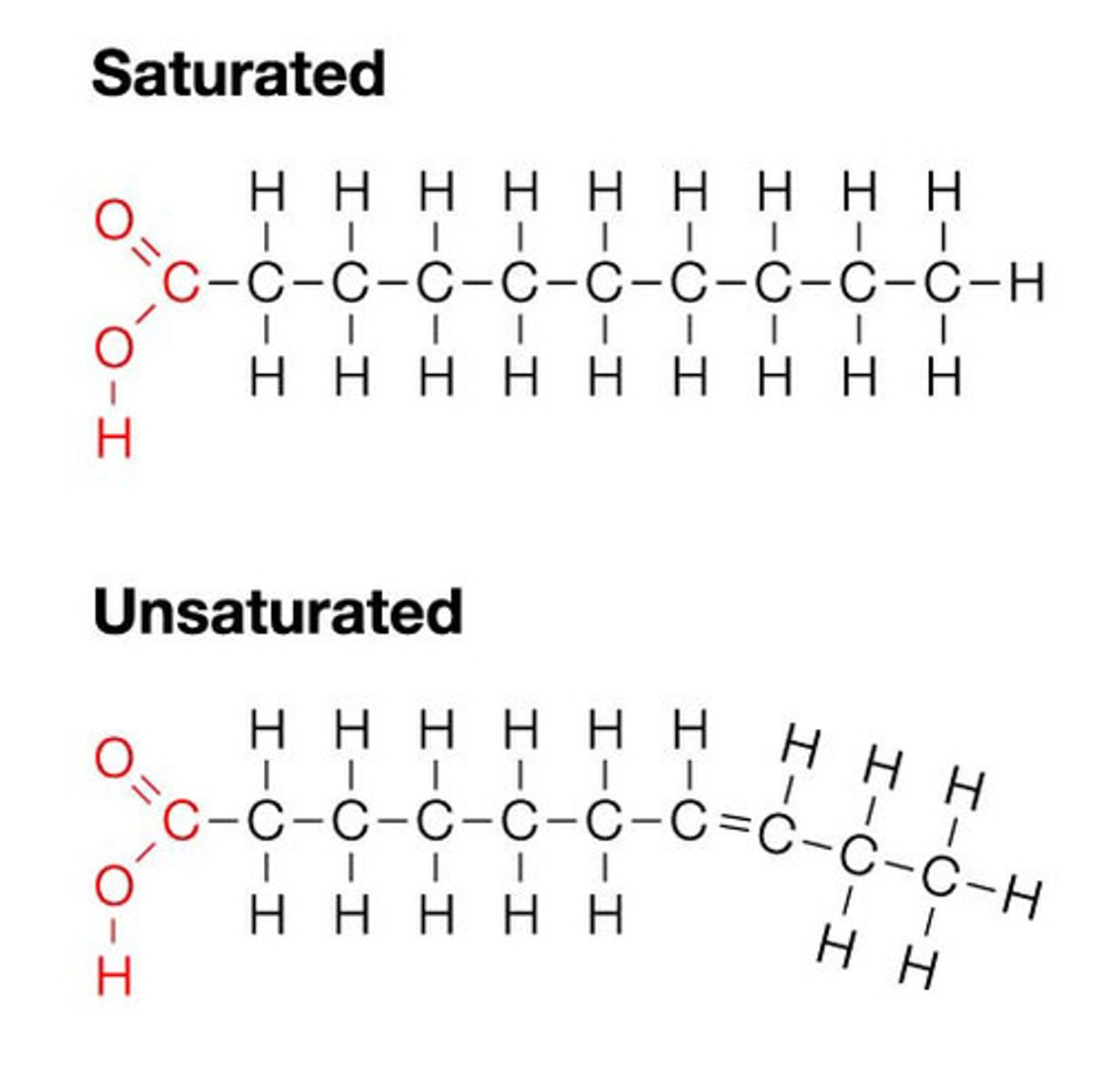
The plants growing in colder weather tend to have more of _____________________ fats in their cell membrane.
unsaturated fatty acids
***bc unsaturated fats melt at a lower temperature, but since it is in cold weather there is no need for saturated fatty acids
Which part of the phospholipids will be exposed if they are suspended in a solvent such as cyclohexane (C6H14) shown below.
the hydrophobic tail
***bc the hydrophilic head will attract the molecule and leave the hydrophobic tail exposed
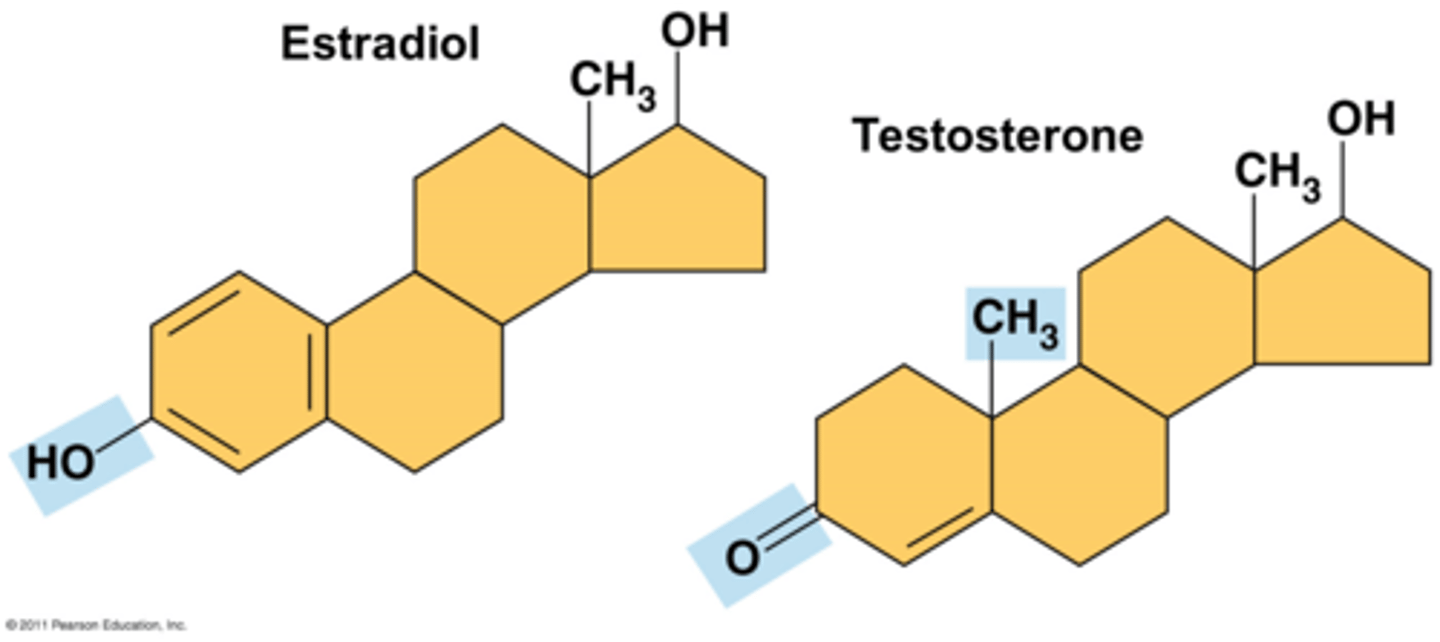
what functional group differentiates testosterone from estradiol
the hydroxyl group
***the molecules are very similar except that testosterone doesn't have a hydroxyl group on the end
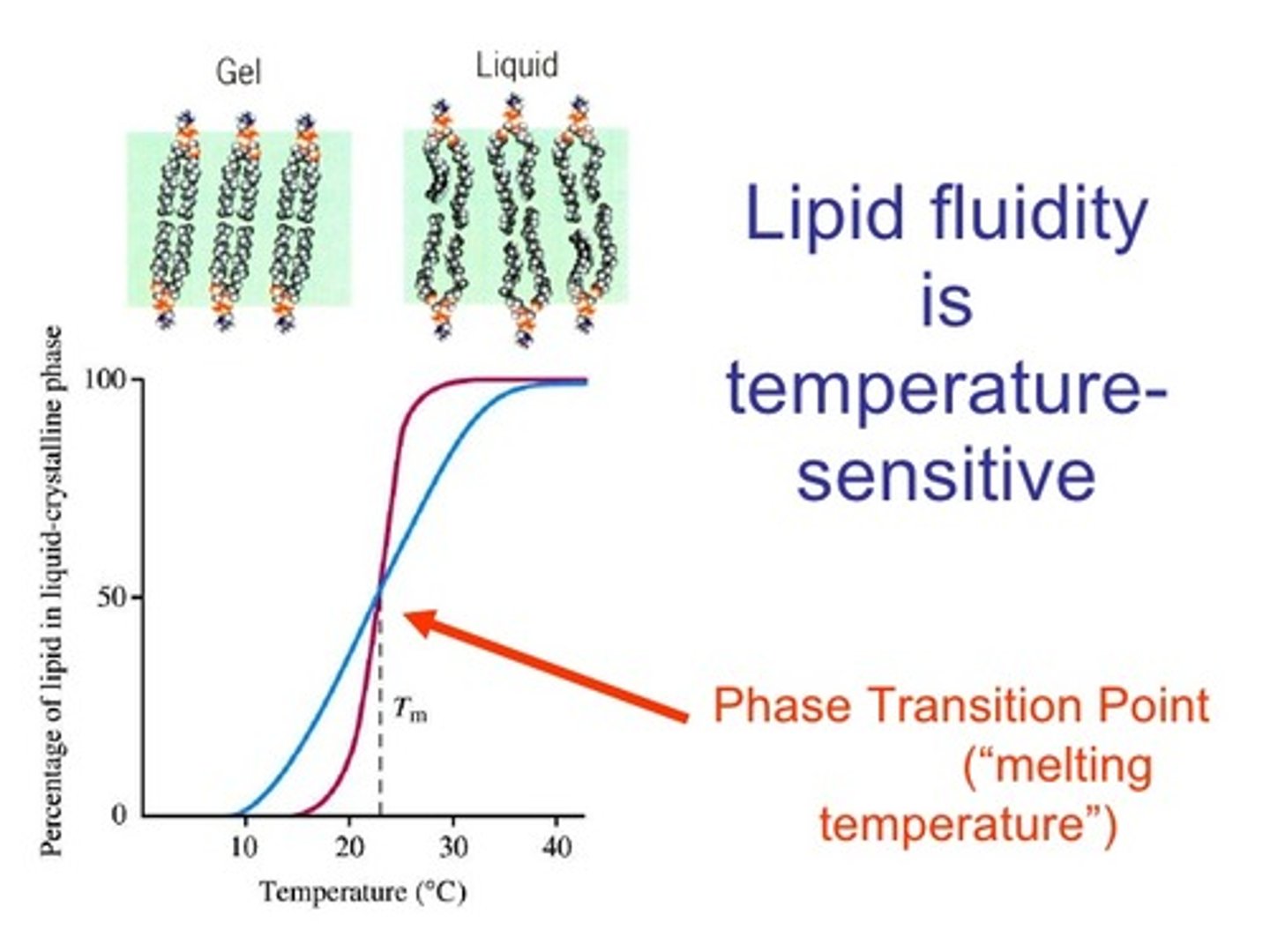
Study the above graph and answer the following question.
The blue green line is with cholesterol and the red line is without cholesterol.
How does cholesterol moderate the fluidity at varying temperatures?
liquid fluidity will be higher as the temperature goes up
***the red line has a more steep incline meaning it will become more fluid faster and easier
Which of the following is NOT a function of proteins?
Serve as genetic material to pass information to next generation
***proteins cannot replicate and serve as genetic material. DNA does.
A protein is made of 100 amino acids. How many peptide bonds are in this protein?
99
***think of N-1
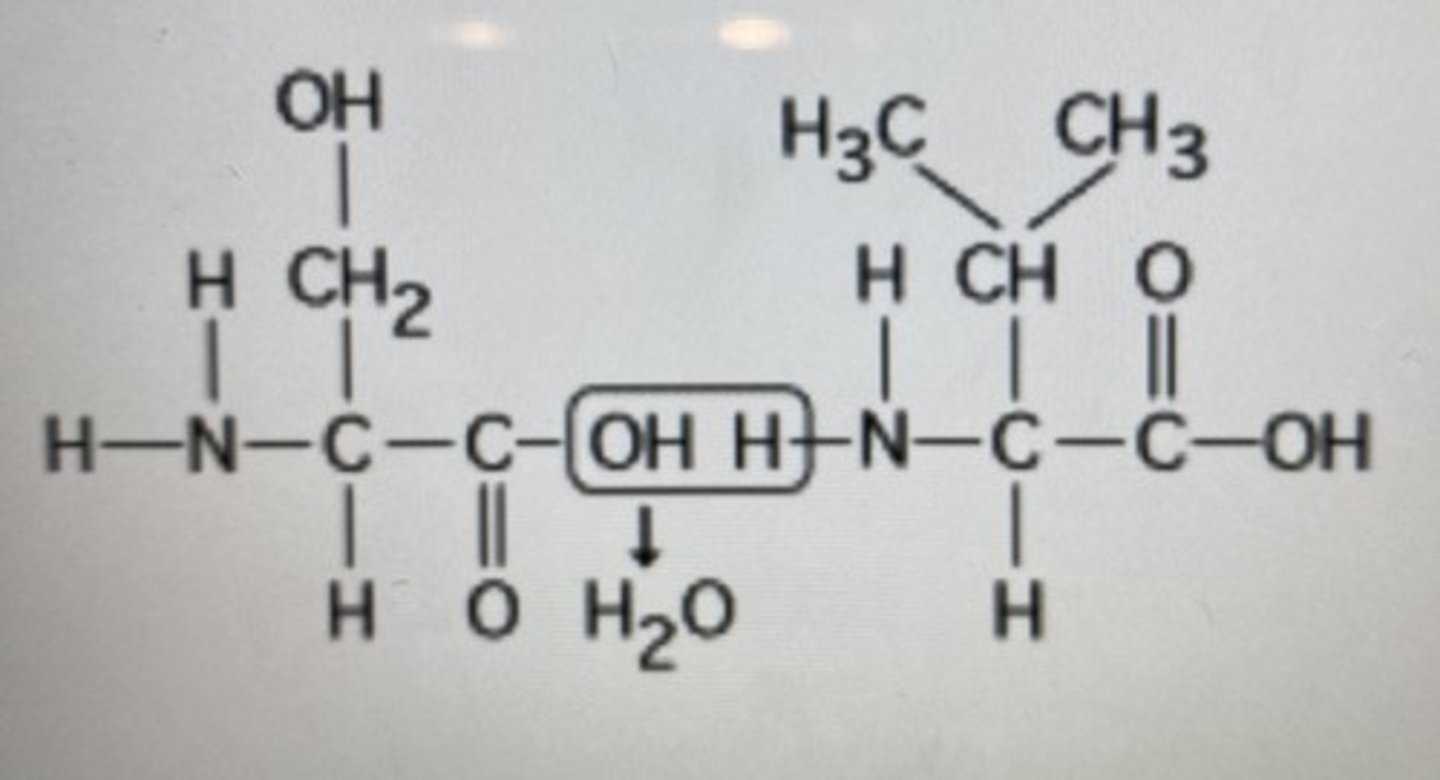
What will be the resulting product(s) of the following condensation synthesis reaction?
water and dipeptide
***hydrolysis creates water and bond brings the two peptides together making them once dipeptide
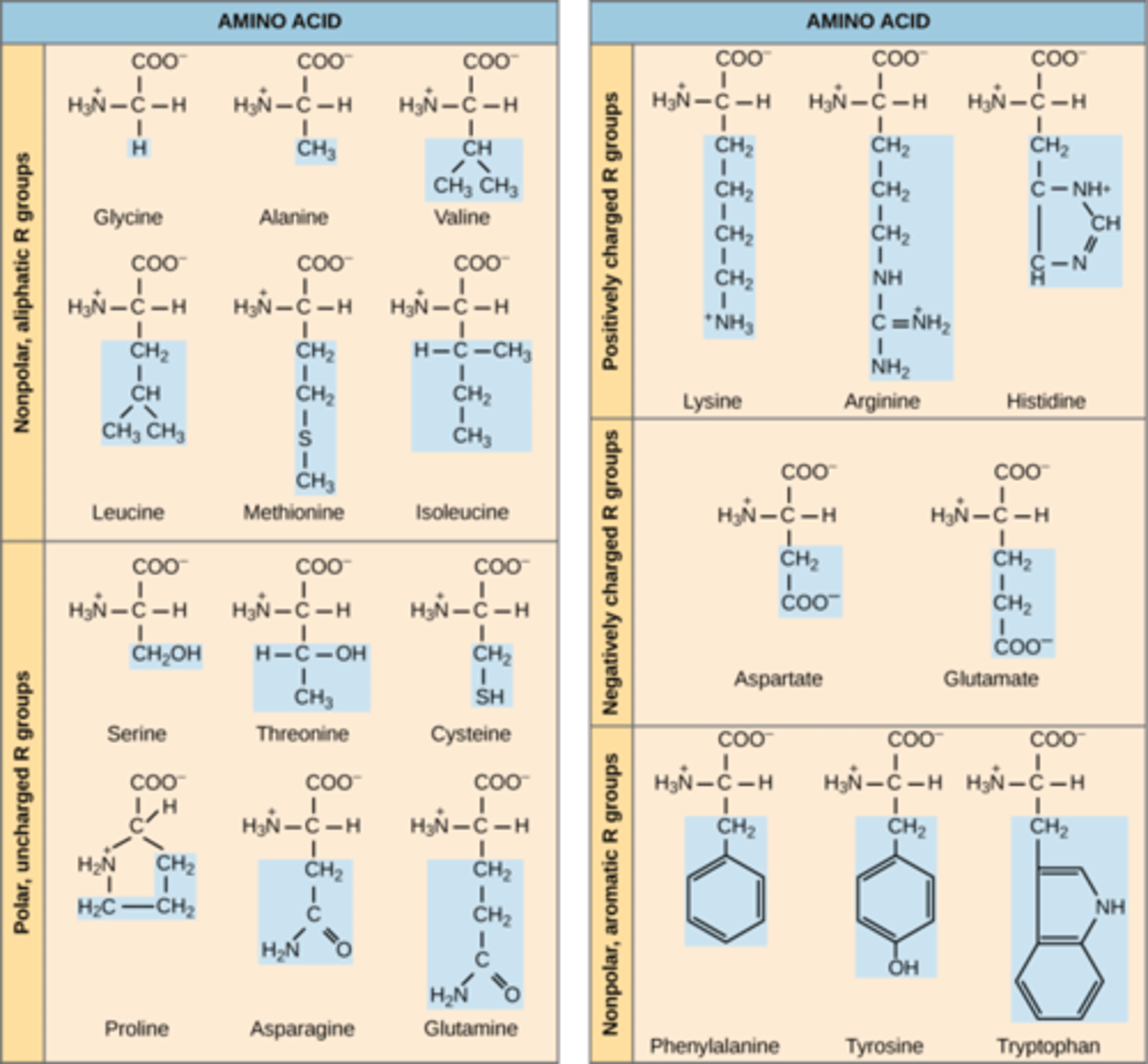
If a mutation in the DNA resulted in changing a critical amino acid from valine (with -CH3 in side chain) to serine (with -OH in side chain), it will make the new amino acid to be on the _______________ part of soluble protein inside cytoplasm.
exterior
***polar molecules will be on the outside that face the aqueous environment
Ribonuclease A (RNase A) contains 4 disulfide (-S-S-) bonds making it stable.
What is the minimum number of cysteine residues with one -SH group in each, in this RNase A?
8
***4 disulfide bonds, 4x2=8
What will be the interaction between the side chains of leucine and isoleucine (both contain only -CH3 in side chain) within a protein surrounded by an aqueous solution?
hydrophobic interactions and Van der Waals
***hydrophobic interactions are the primary form of intermolecular forces between nonpolar molecules. Leu and Ile are nonpolar
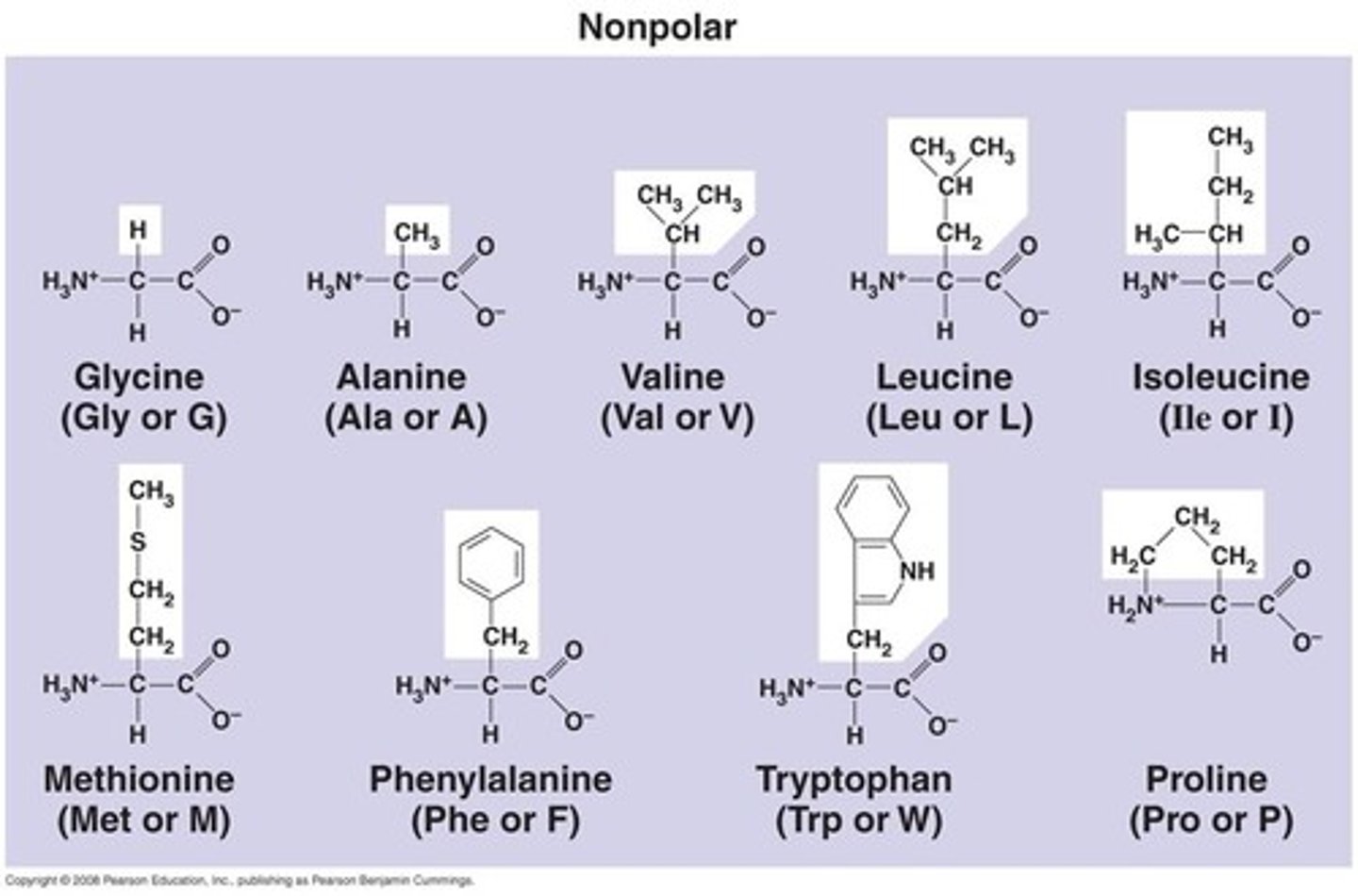
An Amino acid with -NH3+ on its side chain will interact with another amino acid with -COO- in its side chain through _________________ . Answer only the side chain interaction.
ionic bonds
***a positively charged amine group and negatively charged carboxyl group will bond through an ionic bond.
RuBisCO (Ribulose Bisphosphate Carboxylase and Oxygenase) is a complex protein made up of 8 large subunits and 8 small subunits making the whole enzyme complex of approximately 560 kDa (kilo Daltons) in size. How much would 1 mole of this RuBisCO weigh in pounds (1 Kg = 2.2 Lbs.)?
12323
what is used to label (tag) nucleic acid but not proteins?
phosphorus
*Phosphorus in present in DNA and RNA but not in proteins
what is the molecule shown?
it is a nucleic acid DNA with 4 nucleotides connected through phosphodiester linkage
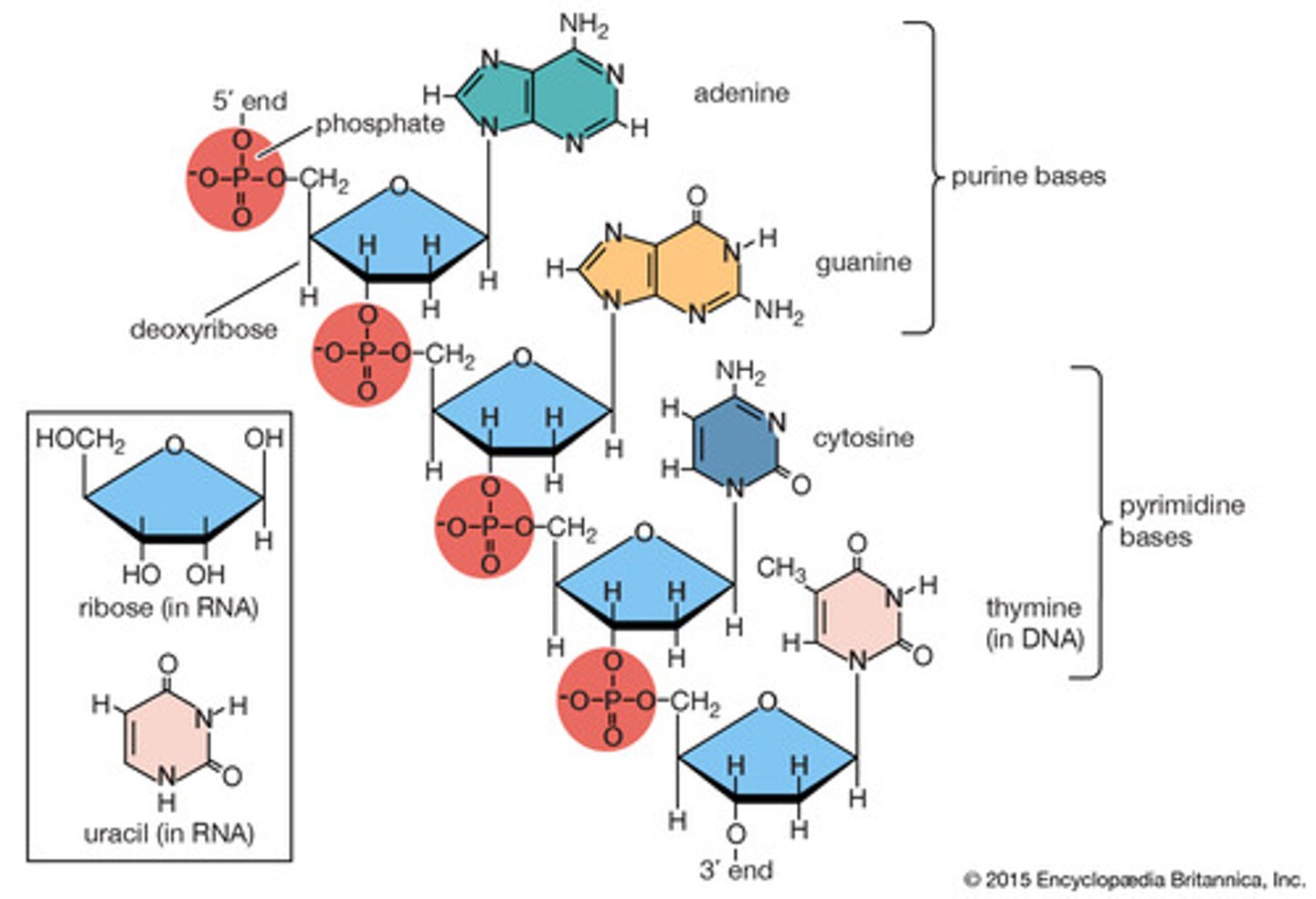
what structure is held together by H-Bonds?
complementary base pairing of RNA strand
**the two strands need to be able to separate, so H-bonds hold them together while they exit the nucleus for translation yet allow them to easily separate for protein synthesis
what type of bonds are holding these nucleotides together?
phosphodiester bonds connect the nucleotides to each strand of DNA
The following DNA sequences were obtained from three DNA fragments that overlap each other in terms of their sequence.
5'-AGATCGATT-3'
5'-TCCGGATCG-3'
5'-GATTGTCCGG-3'
Identify the overlaps and the correct sequence of assembled DNA.
5'-AGATCGATTGTCCGGATCG-3'
Which of the following, if changed significantly, would affect all the others?
DNA
**DNA is the template for RNA synthesis (transcription) and protein synthesis (translation)
Proteins cannot replicate but DNA can replicate because it can form ________ that is not possible by proteins
complementary base pairings
**explained by chargaff's rule: the nucleotide base pairings allow DNA to break apart and form new bonds.
Proteins can catalyze reactions while DNA cannot because proteins contain _______ which is not present in DNA.
variable secondary structures
**secondary structures include Alpha-helix and B-pleated sheet
Cell Theory in its modern form includes all of the following postulates except __________________
all cells contain nucleus
***prokaryotes don't have a nucleus, duh
You would be wasting your time if you were trying to use a light microscope to see_______
Viruses
***they are too small to see through a light microscope, you would need an electron microscope
Which of the following molecules do not have any charges on them so that they cannot be separated by gel electrophoresis? There may be more than one answer.
Carbohydrates and Triglycerides
***these two molecules have to be separated by different methods
In agarose gel electrophoresis, DNA or RNA fragments migrate towards ___________ end of the gel plate because of their____________.
the anode ..... charge
***DNA is negatively charged so it will be repelled from the cathode
Arrange the following sequence of procedures in the proper order in this experiment done to determine proteins present in pea plants during germination.
1. Load the proteins in polyacrylamide gel electrophoresis and separate them
2. Break the cells by grinding them in liquid nitrogen and suspending in a buffer
3. Select a suitable tissue to isolate proteins expressed only during germination
4. Centrifuge the broken cells to purify proteins from other molecules
3,2,4,1
***First, identify where the cells come from. Then break the cells to retrieve proteins and centrifuge the components to isolate the proteins. Then load the gel with the proteins and run it to separate according to size and charge.
Lipids and carbohydrates do not have any charges associated with them, making it difficult to separate them by gel electrophoresis. What methods are good for separating such molecules?
MS, GC, TLC, HPLC
*as mentioned before
A sewage line broke and contaminated drinking water source of a rural town. You are asked to investigate if there is any bacterial contamination after fixing the sewage leak. What methods would you follow?
collect a water sample and observe it under a light microscope to find if there are bacteria
***bacteria can be observed through a light microscope
You are working for CDC and investigating a toxic E. coli contamination in lettuce.
Which method(s) would be useful to definitely identify the toxic E. coli?
use a light microscope to figure out which piece of lettuce is contaminated. then use a marker to label the toxic chemical so you can see if it is toxic or not
You have isolated some organisms that are unicellular with cell wall containing peptidoglycan and have flagella without any membrane enclosure. Which group do these organisms belong to?
Bacteria only
**see table
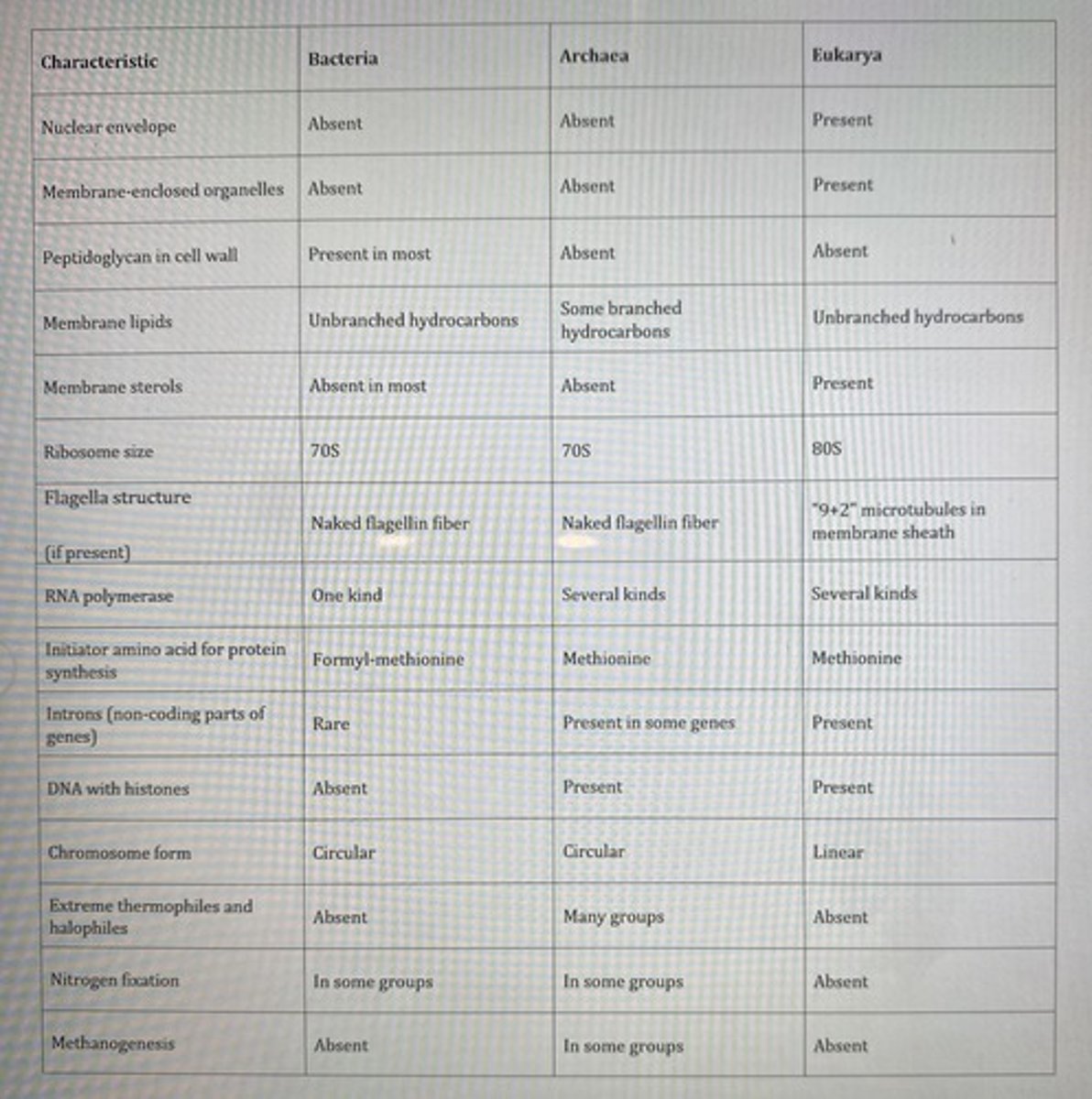
Which organisms will have linear chromosomes with histone proteins bound to their DNA?
Eukarya only
**see table
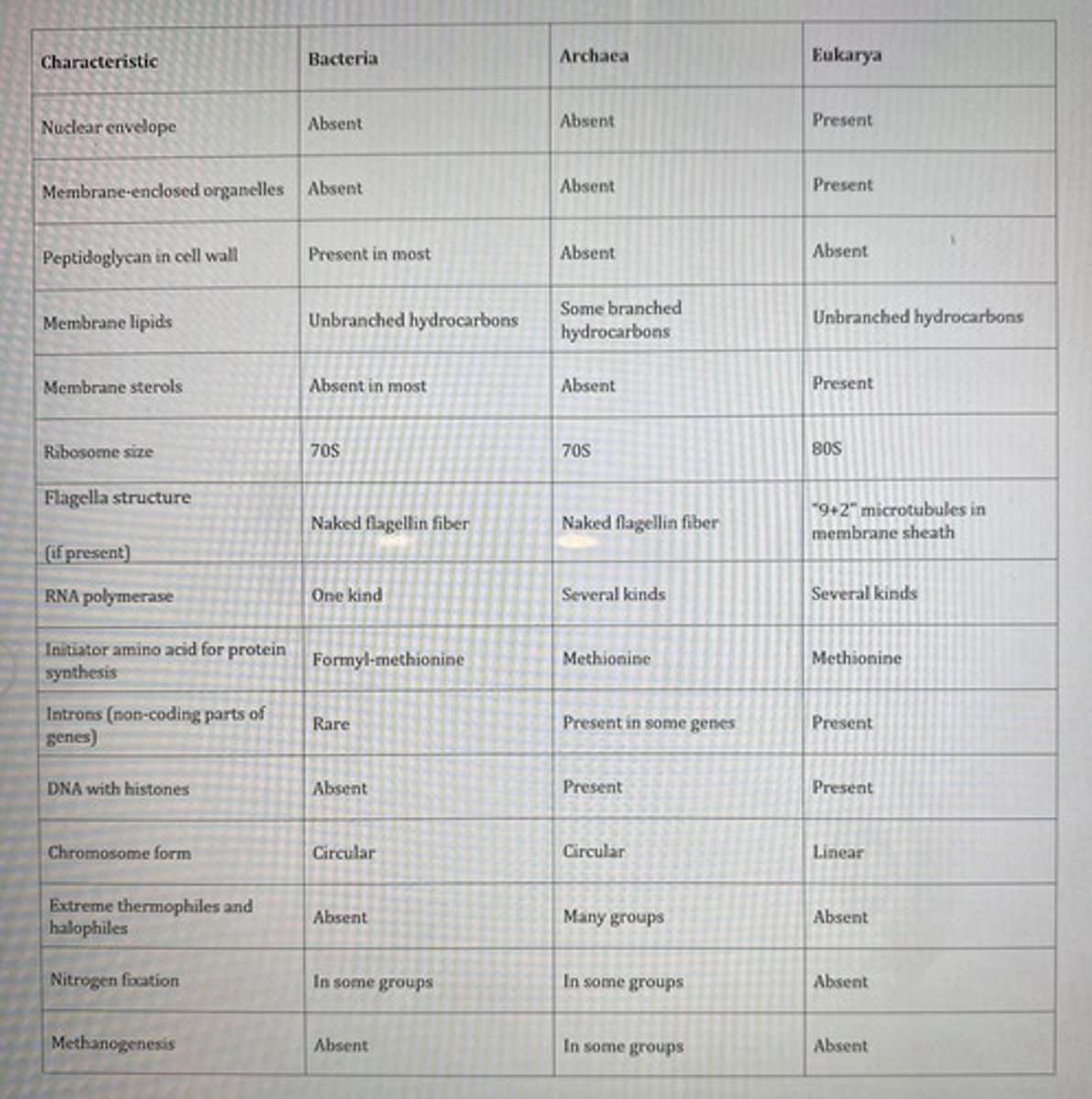
Which of the following is the most rapidly reproducing organisms, based on their simplicity or complexity?
bacteria only
**read chapter 5... jk see the table
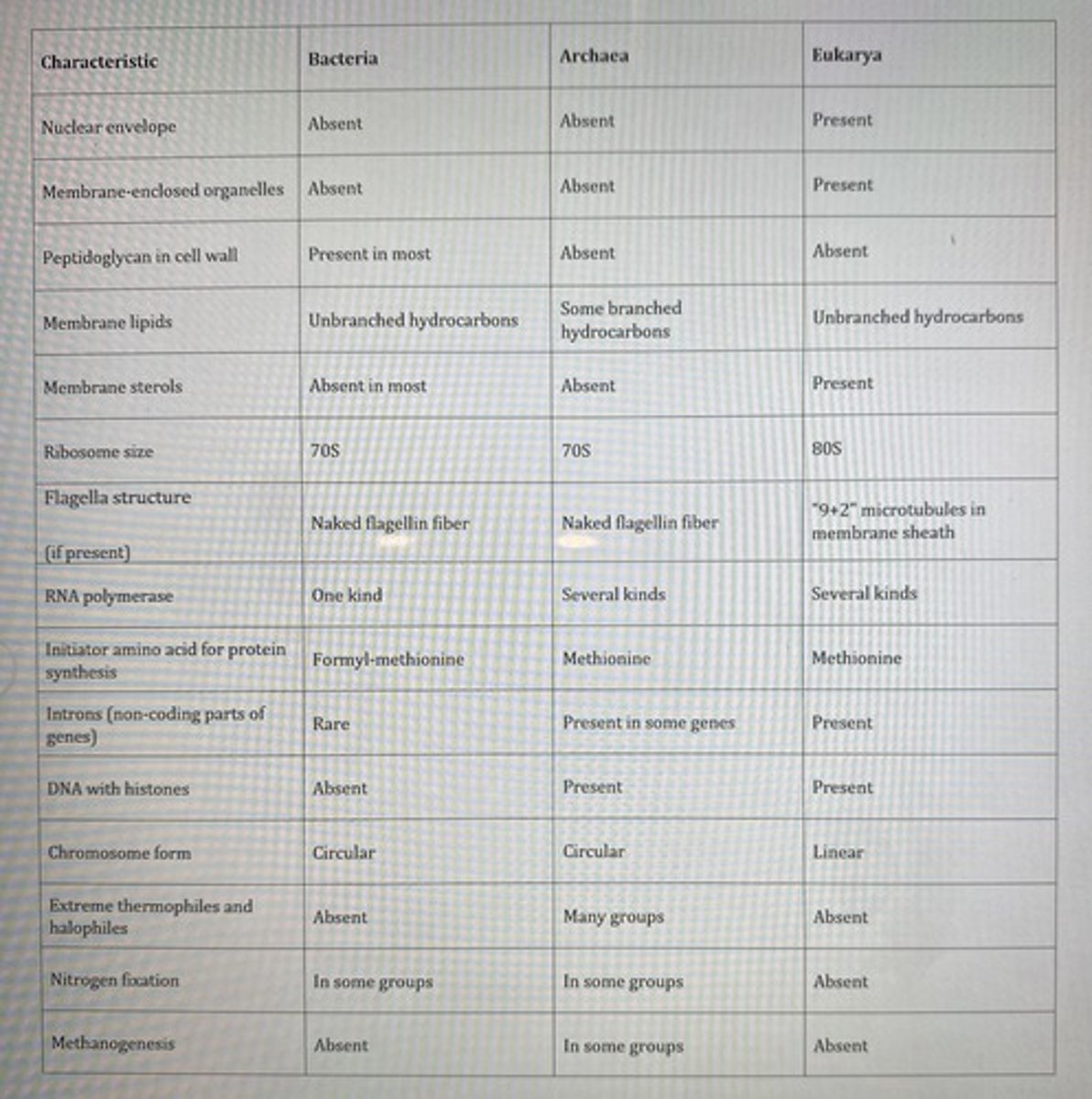
How many different types of ribosomes are found in a plant cell? Hint: consider the various locations of the plant genome.
3
How does a larger cell compensate for the reduced amount of surface area as the cell volume increases?
compartmentalization
***allows more surface area while keeping the same volume
Tasmanian tigers are extinct because of hunting by humans but the close relative Tasmanian devils are still alive and thriving well.
If you were to use an adult cell's nucleus with intact DNA from a Tasmanian tiger to replace the nucleus of a Tasmanian devil's egg cell and make it produce to a new organism in a surrogate Tasmanian devils womb, what kind of organism will it make?
Mostly Tasmanian Tiger with the mitochondrial DNA from the T. Devil
Read this paragraph and answer the question
CYP3A4 is a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases that catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids, and other lipids components.
The CYP3A4 protein localizes to the endoplasmic reticulum, and its expression is induced by glucocorticoids and some pharmacological agents. Cytochrome P450 enzymes metabolize approximately 60% of prescribed drugs, with CYP3A4 responsible for about half of this metabolism; substrates include acetaminophen, codeine, ciclosporin (cyclosporin), diazepam, and erythromycin. The enzyme also metabolizes some steroids and carcinogens. Most drugs undergo deactivation by CYP3A4, either directly or by facilitated excretion from the body.
If someone is addicted to Oxycontin, shown below, which of the following enzymes and structures would try to detoxify them?
cytochrome p450, smooth ER
***don't be lazy, read
When the pH was increased from 5 to 7 in an in vitro experiment, these structures purified from animal cells stopped digesting their contents. What are these structures?
lysosomes
***lysosomes are what digest contents
The major differences in the function of rough ER compared to smooth ER is to __________________
make proteins
***rough ER has ribosomes
Which of the following pathway is the most probable pathway for secretory proteins that is glycosylated along the way?
Rough ER → vesicles → Golgi apparatus → plasma membrane
***proteins are made so they have to come from the Rough ER
How many cellular structures the rough endoplasmic reticulum is associated with?
8
Fresh water protists such as paramecium maintain homeostasis of its salt concentration by pumping water out of the cell. Which of the following structures would serve this purpose?
Contractile vacuole
***
Authored by Benjamin L.WeisaEnricoSchleiffabWilliamZergesc 2012
Cells have complex membranous organelles for the compartmentalization and regulation of most intracellular processes. Organelle biogenesis and maintenance requires newly synthesized proteins, each of which needs to go from the ribosome translating its mRNA to the correct membrane for insertion or translocation to an organellar subcompartment. Decades of research have revealed how proteins are targeted to the correct organelle and translocated across one or more organelle membranes to the compartment where they function. The paradigm examples involve interactions between a peptide sequence in the protein, localization factors, and various membrane-embedded translocation machinery. Membrane translocation is either cotranslational or posttranslational depending on the protein and target organelle. Meanwhile, research in embryos, neurons, and yeast revealed an alternative targeting mechanism in which the mRNA is localized and only then translated to synthesize the protein in the correct location. In these cases, the targeting information is encoded by cis-acting sequences in the mRNA ("Zipcodes") that interact with localization factors and, in many cases, are transported by molecular motors on cytoskeletal filaments. Recently, evidence has been found for this "mRNA-based" mechanism in organelle protein targeting to the endoplasmic reticulum, mitochondria, and the photosynthetic membranes within chloroplasts.
mRNA sequences
What will happen if the segment of DNA coding for the target sequence is deleted from a gene encoding a protein located in lysosomes?
it will not reach the target site, lysosome
***it erases the memory that it has to go to the lysosome
Which of the following is not a common feature of both chloroplast and mitochondrion?
Makes essential amino acids
***Essential amino acids are made only by chloroplasts as we need to consume them in our diet.
Which of the following action facilitated by microfilaments, a plant cell will not be able to do?
Cleavage furrow (pinching in middle of cell) formation during cell division
***The tough cell wall prevents cells from cleaving.
We need to consume essential amino acids in our diet because we do not have ______________ that make essential amino acids.
Plastids
***Plastids include chloroplasts, leucoplasts which make essential amino acids
Which of the following structures are important for providing energy for cytoplasmic functions in a plant?
mitochondrion
***Mitochondrion makes ATP for the cellular functions.
How does the double membrane structure of mitochondrion help in its function to make ATP?
Maintaining conditions within and between the two membranes to allow ATP synthesis
***The pH of the mitochondria is very important for ATP synthesis and internal and external conditions need to be monitored closely for optimal functioning.
Both mitochondria and chloroplast are maternally inherited. Some plants develop resistance to atrazine herbicide through a mutation of a gene located in the chloroplast. If male plants without any such herbicide resistance are crossed with an atrazine resistant female plant, what will be the resistance level of offspring plants?
All offspring will be resistant.
***Since mitochondria and chloroplasts are only inherited from the mother, all offspring will have the genes located on those organelles.
Read the following paragraph from https://ghr.nlm.nih.gov/mitochondrial-dna and answer. What types of symptoms would show in an individual with a defective mitochondrial genes?
Mitochondria are structures within cells that convert the energy from food into a form that cells can use. Each cell contains hundreds to thousands of mitochondria, which are located in the fluid that surrounds the nucleus (the cytoplasm). Although most DNA is packaged in chromosomes within the nucleus, mitochondria also have a small amount of their own DNA. This genetic material is known as mitochondrial DNA or mtDNA. In humans, mitochondrial DNA spans about 16,500 DNA building blocks (base pairs), representing a small fraction of the total DNA in cells.
Mitochondrial DNA contains 37 genes, all of which are essential for normal mitochondrial function. Thirteen of these genes provide instructions for making enzymes involved in oxidative phosphorylation. Oxidative phosphorylation is a process that uses oxygen and simple sugars to create adenosine triphosphate (ATP), the cell's main energy source. The remaining genes provide instructions for making molecules called transfer RNA (tRNA) and ribosomal RNA (rRNA), which are chemical cousins of DNA. These types of RNA help assemble protein building blocks (amino acids) into functioning proteins.
low energy overall
Give an example of how cytoskeletal elements helps in sexual reproduction of animals.
microtubules can contribute to cell shape and motility, for example in sperm the shape and tail are used to be able to penetrate the female ovum
Which of the following is the correct sequence of plant cell structural layers, beginning with the cytoplasmic side and progressing outward? It may help to draw two adjacent plant cells and the cell walls to answer this question.
Plasma membrane, secondary wall, primary wall, middle lamella
***The innermost structure is the plasma or cell membrane. Then it is the secondary cell wall, then the primary cell wall which is the toughest and most exterior portion of the cell. The middle lamella is jelly-like and connects two adjacent cells together.
If you were to genetically engineer a papaya plant that will resist fungal disease, you would like to express high levels of the enzyme _____________ that will degrade the fungal cell wall.
chitinase
***Chitin is what fungal cell walls are made out of. Chitinase degrades chitin
What structures are similar to each other in the list given below?
Plasmadesmata and gap junctions
***Both connect adjacent cells through small pores through the plasma membrane or cell wall
What are the barriers for glucose to enter a bacterial cell from the medium?
capsule --> cell wall --> plasma membrane
Bacteria and fungi can degrade wood because they can make the enzyme _________________________.
cellulase
***since plants use cellulose, the cellulase is what degrades cellulose
Which of the following structures are important for maintaining water pressure and movement of water in a tall plant?
cell wall
***provides a rigid structure that can create an osmotic pressure to push water to a cell with less water
The digestive tract lining contains epithelial (outer lining) cells that do not allow food particles to randomly go inside the body except through specific proteins on their cell membrane. Which of the following structure blocks random diffusion of food particles between epithelial cells?
Tight junctions
***Fuse 2 cell membranes through integral membrane proteins (prevents any solutes from moving between the two cells)
While you swim in the pool, you do not absorb water and swell because the skin has_________________________.
tight junctions and selective permeability
All the following are present in the biological membrane except_____________________
oligosaccharides and polysaccharides
***Polysaccharides are present in cell wall and not in cell membrane
Which of the following molecules will rapidly diffuse through lipid part of cell membrane?
Water and CO2
**Steroid being a non-polar molecule will easily diffuse through the non-polar lipid part of biological membrane
What factors affect the diffusion of molecules in aqueous surroundings?
size and polarity
Which of the following components of cell membrane affects the selective permeability?
phospholipids and protein only
***Not necessarily, they have other means of transport.
Which of the following will affect the fluidity of a cell membrane?
sterols and saturation levels of fatty acid side chains
***Saturation levels of fatty acids will affect fluidity
Use the following information and Table to answer the question.
Five dialysis bags (the synthetic membrane that do not allow glucose to go through) with various contents were placed in beakers with aqueous solutions. The bags were weighed before and after placing them in the beaker for 24 hours.
Bag
Dialysis Bag Content
Beaker content
A
0.5 M glucose
Water
B
0.75 M glucose
0.5 M glucose
C
1.0 M glucose
Water
D
0.75 M glucose
1.0 M glucose
E
Water
1.0 M Glucose
Which bag will lose the most amount of weight (net) after 24 hours incubation in the respective solutions?
bag E
***Water is the most hypertonic of the solutions. The bag with water would lose the most weight as the water goes into the 1.0 M Glucose, which is the most hypotonic solution.
Which of the following molecules will be able to cross a synthetic dialysis membrane with a pore size of 100 daltons (AMU) and not through biological membrane by simple diffusion?
NaCl
***
When freshwater plant cells are placed in sea water the cells will __________________________
get plasmolyzed with water from cytoplasm leaking out.
***Plasmolysis is when the cytoplasmic fluid is leaking out of the cell. This can happen when the cell goes into a hypotonic solution, such as sea water.
Which of the following is/are a highly specific mechanism(s) of cell transport? More than one answer possible, select one.
active transport and receptor mediated endocytosis
***both use proteins to allow things to pass or a specific signal
Which of the following cells can perform phagocytosis? Choose all that apply.
white blood cells and amoeba
***white blood cells absorb foreign substances, and ig the amoeba sisters too
Ion channel proteins are always ____________________________
integral membrane proteins
***Transmembrane proteins are integral proteins that can form a channel for ions to go through the membrane.
Animal viruses infect specific host cells and do not cross host range. This is because viruses are recognized and allowed inside cells through _______________________
Receptor mediated endocytosis
***receptor proteins are recognized on viruses and it doesn't allow them to pass by
Refer to the ECG chart segment shown above and choose the point which represents the depolarized state that results in the action potential.
R
***R represents the most positive membrane potential and leads to the action of reversal to make the membrane potential negative.
Refer to the ECG chart segment shown above and answer the following question. Which proteins will be responsible for the depolarization from Q to R?
Voltage gated Na+ channel pumping Na+ inside
***