Chapter 3: Atoms
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
Electromagnetic Radiation
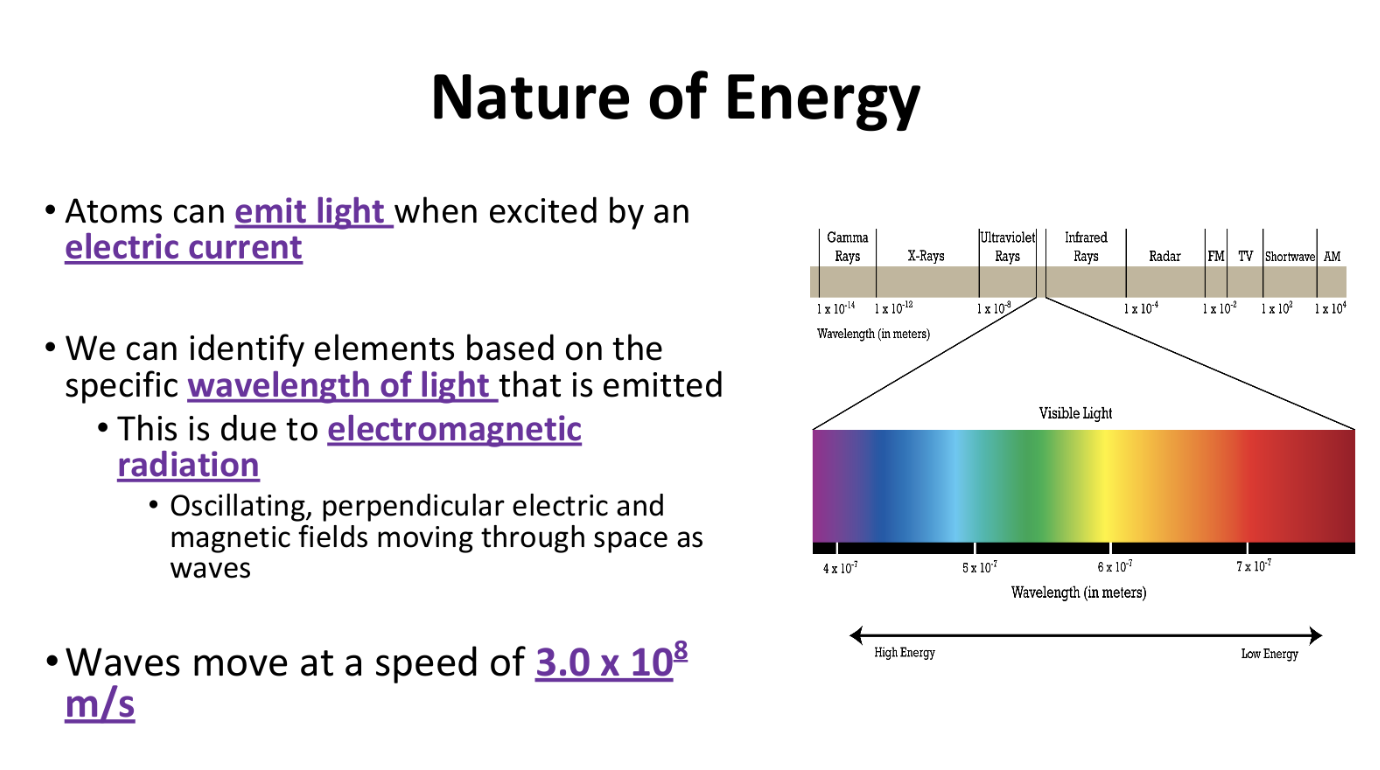
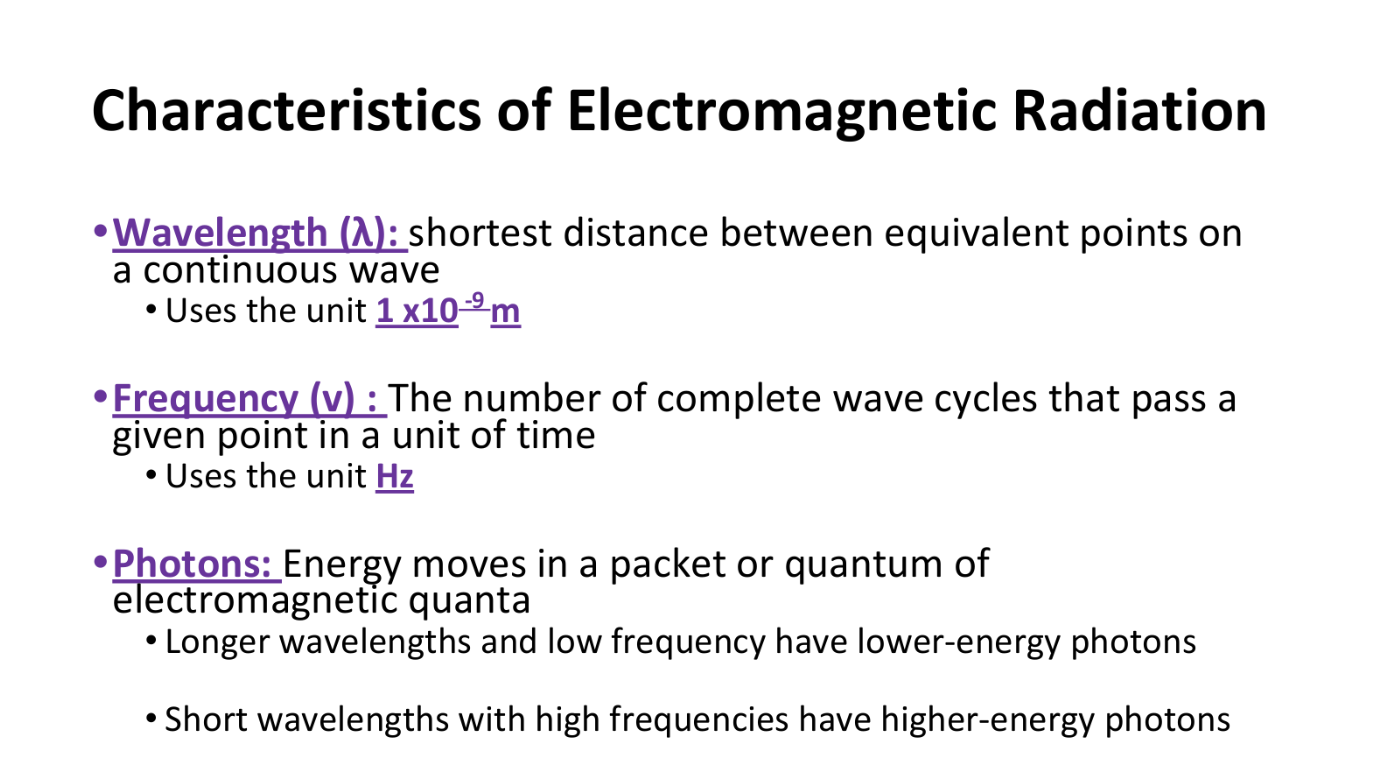
Characteristics of Electromagnetic Radiation
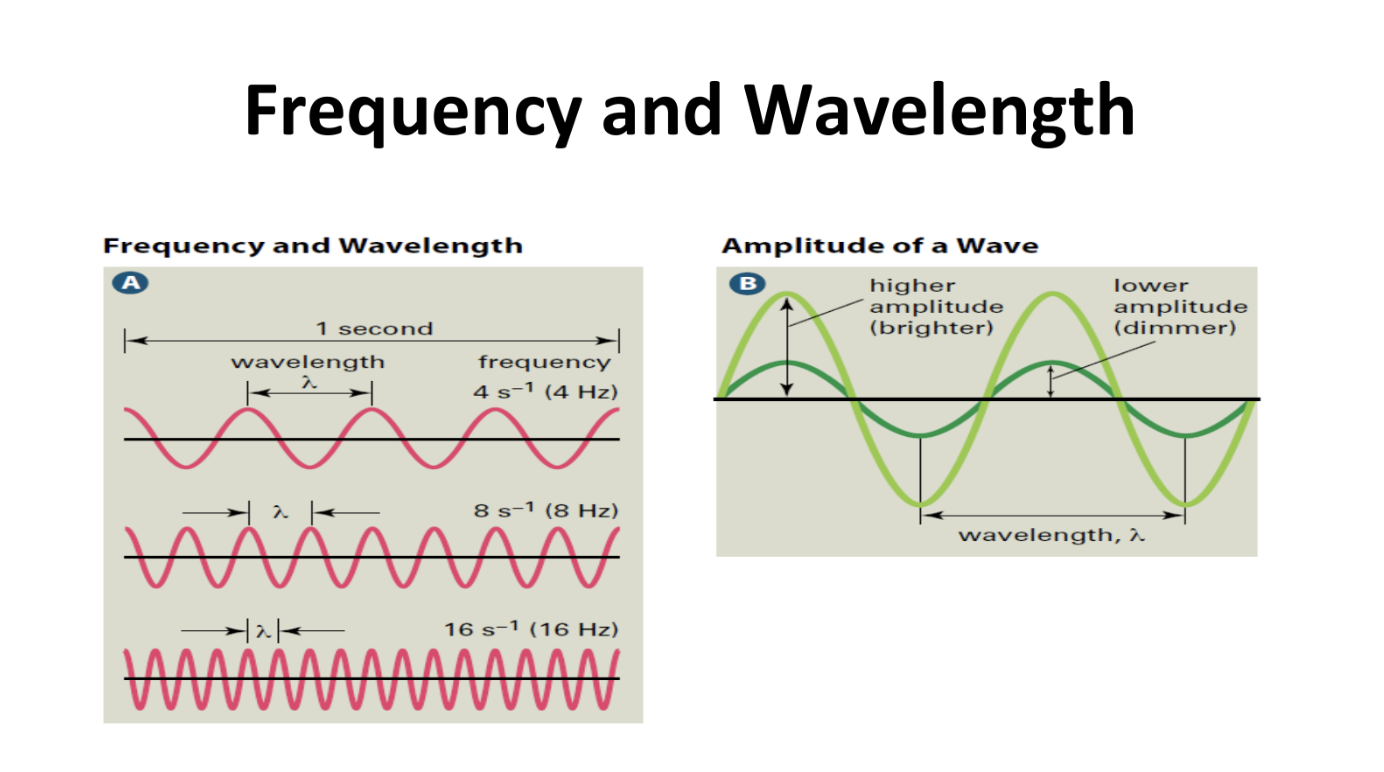
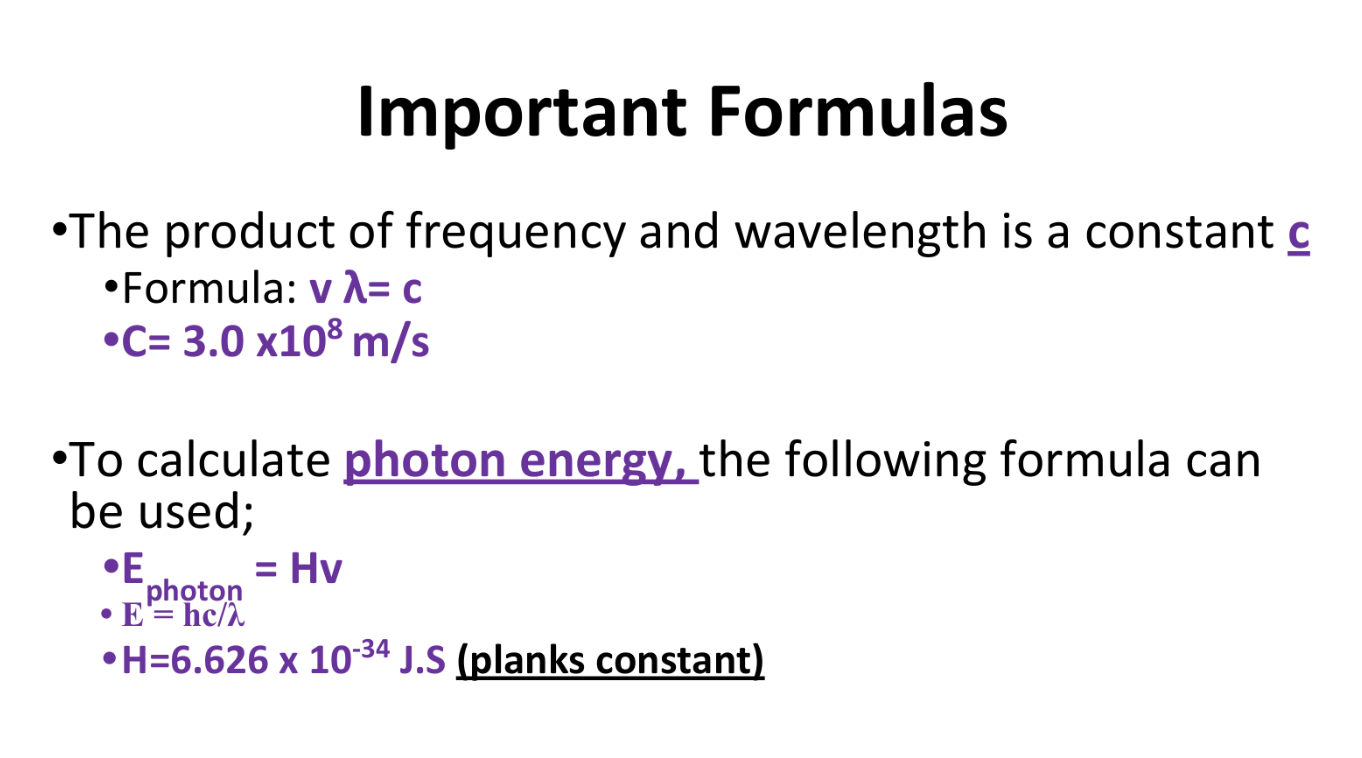
Wave Speed and Photon Energy Equations
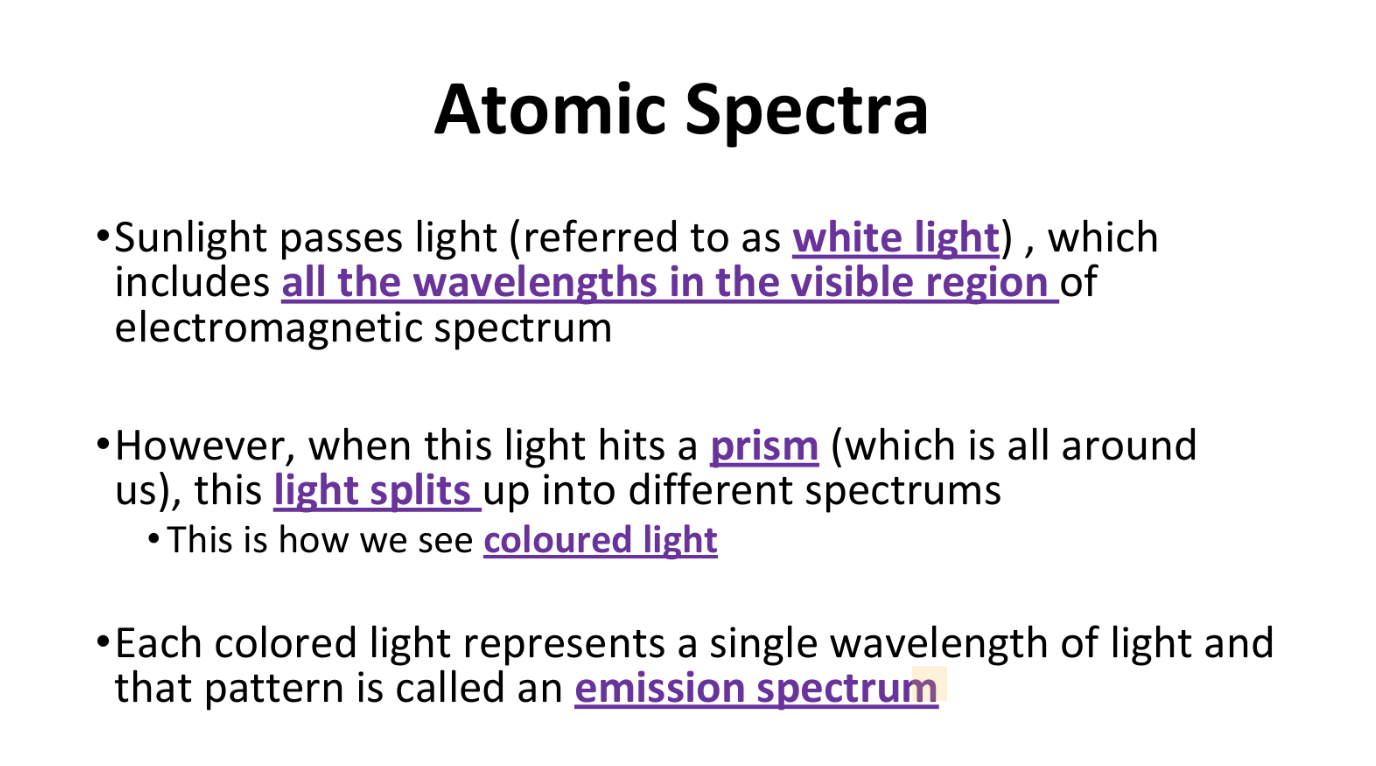
How do we see coloured light? What is an emission spectrum?
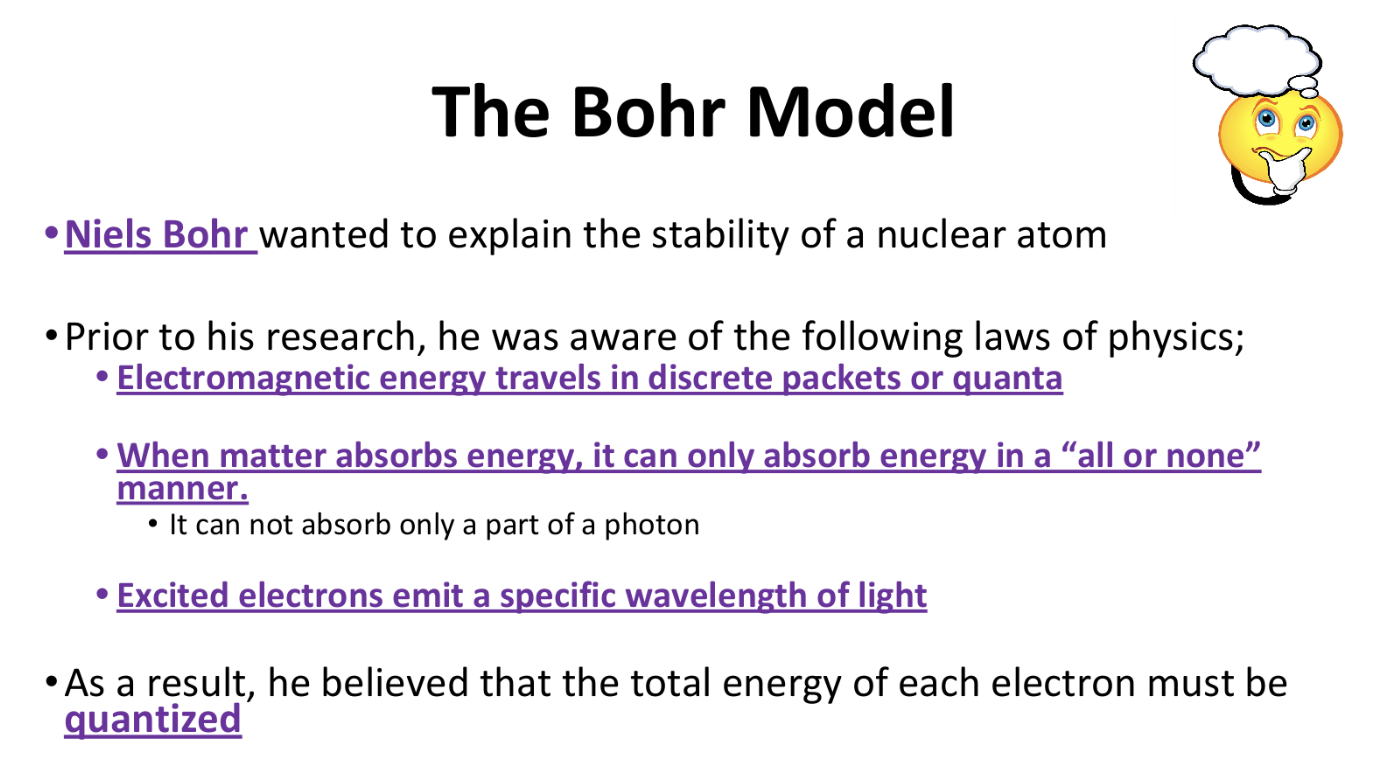
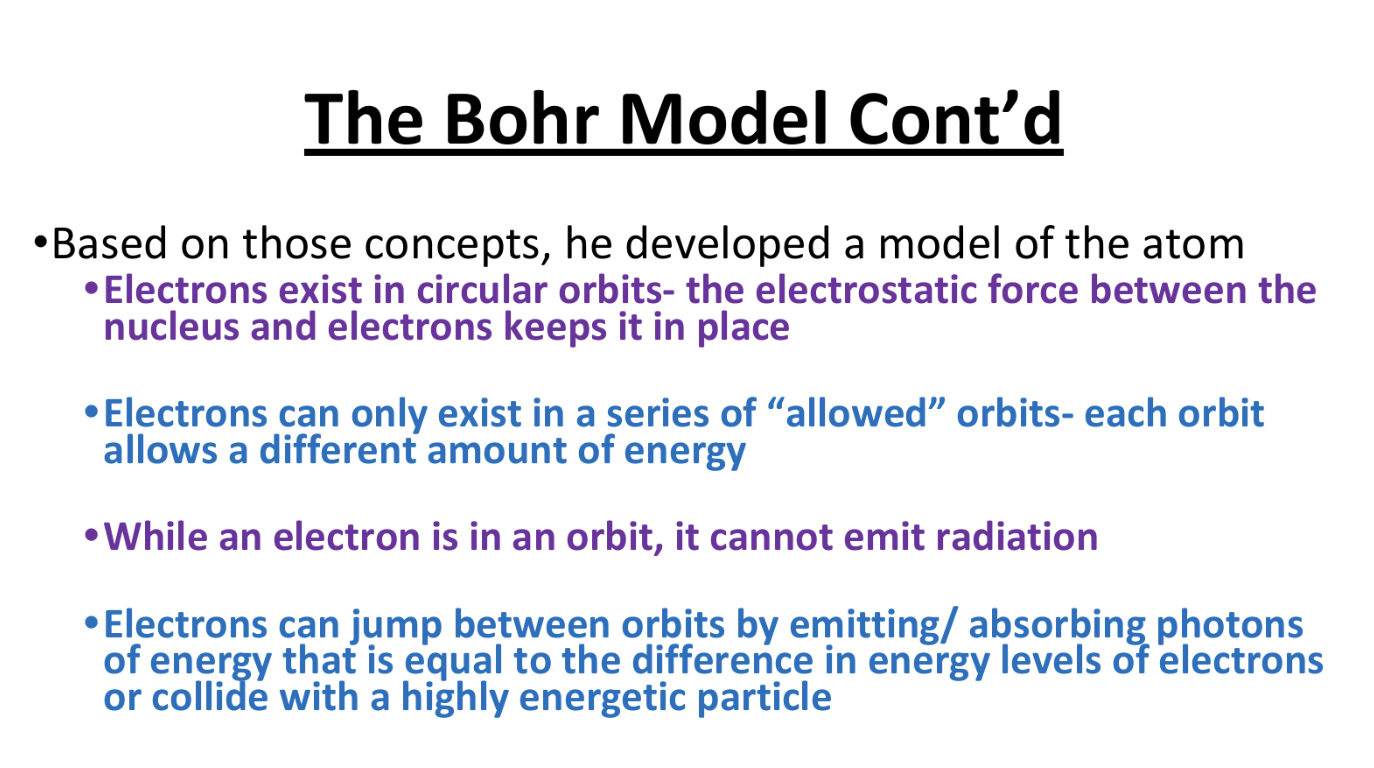
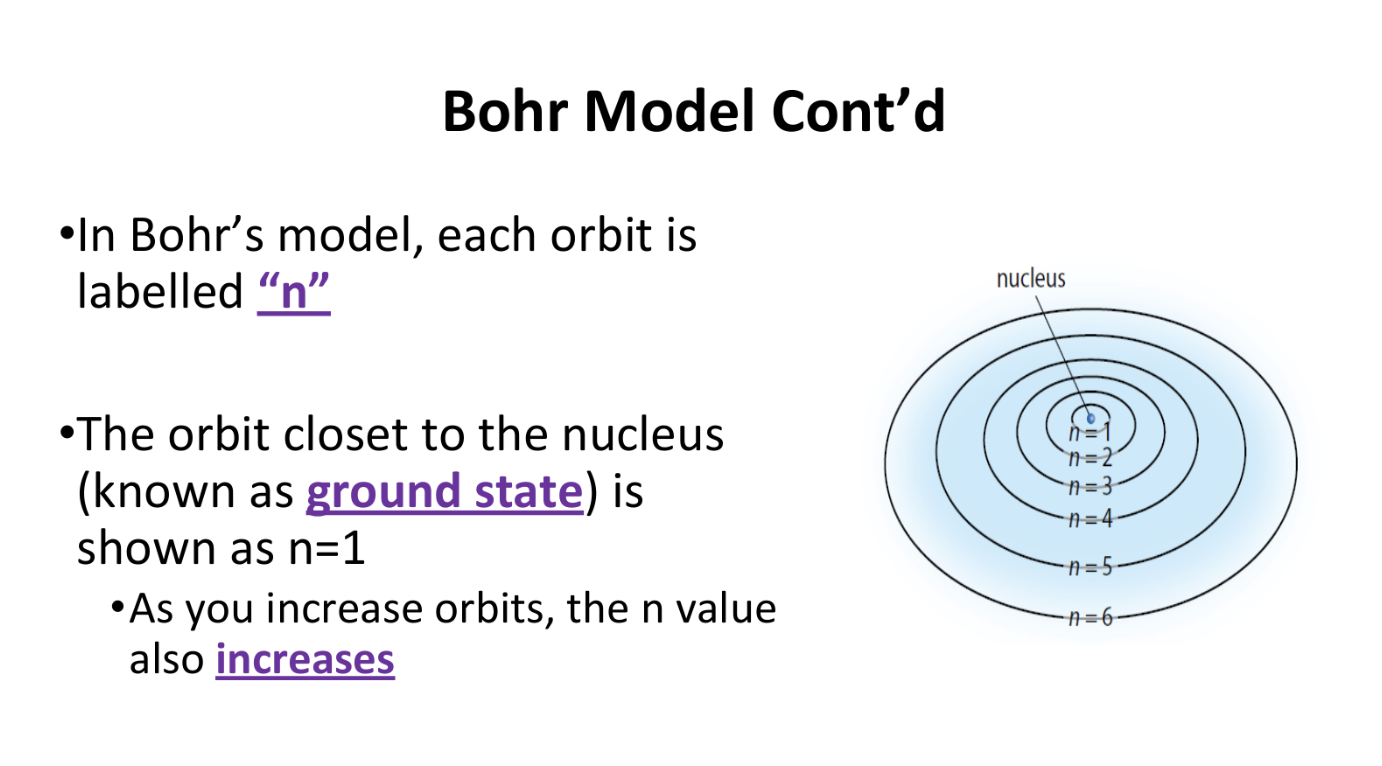
The Bohr Model
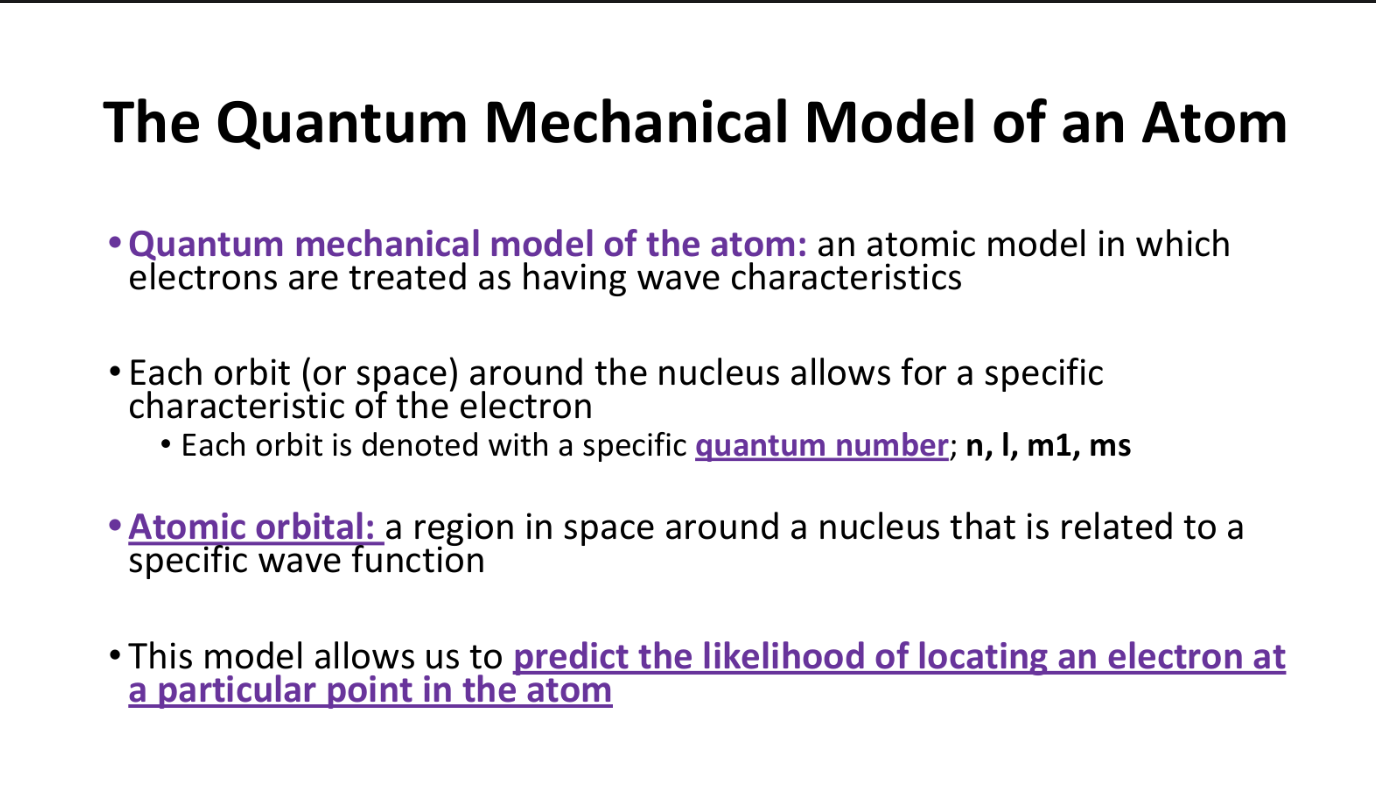
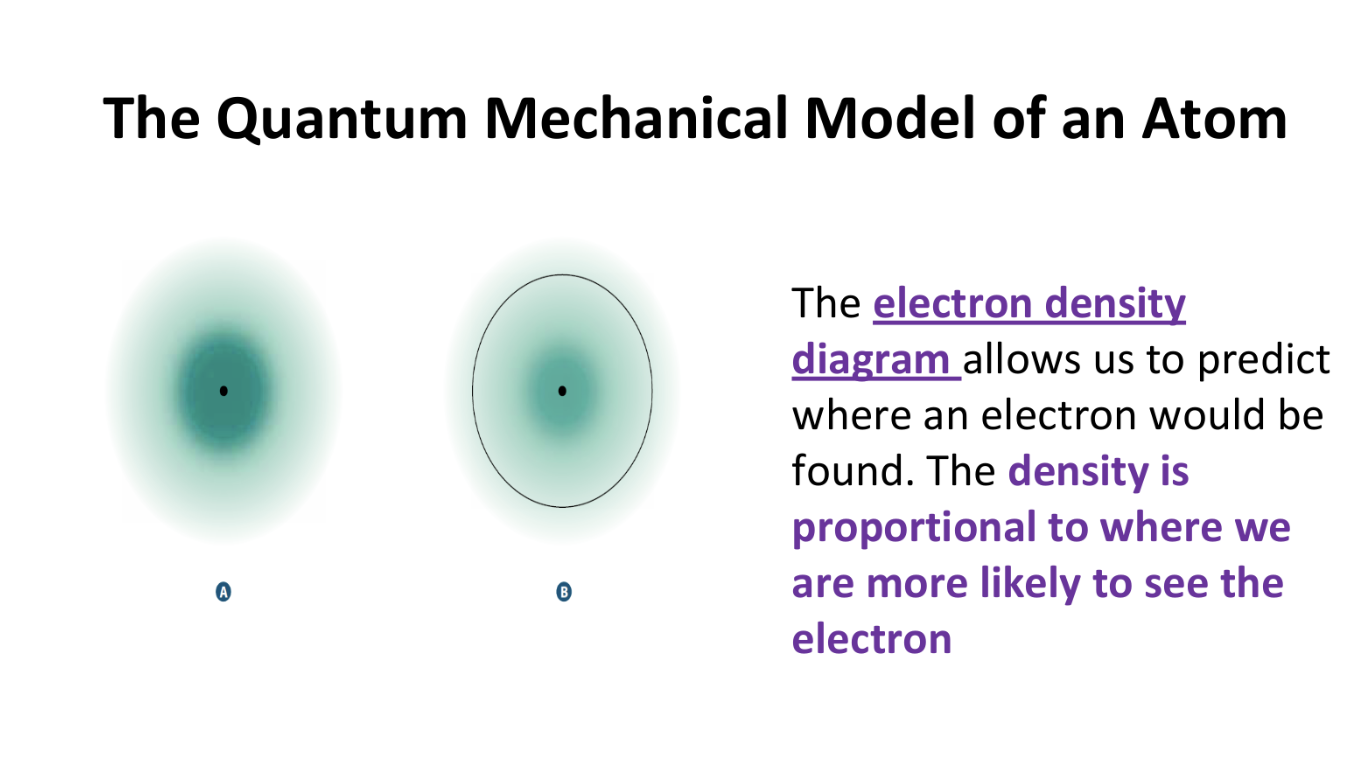
Quantum Mechanical Model of the Atom
Democritus

John Dalton

Dalton’s Model

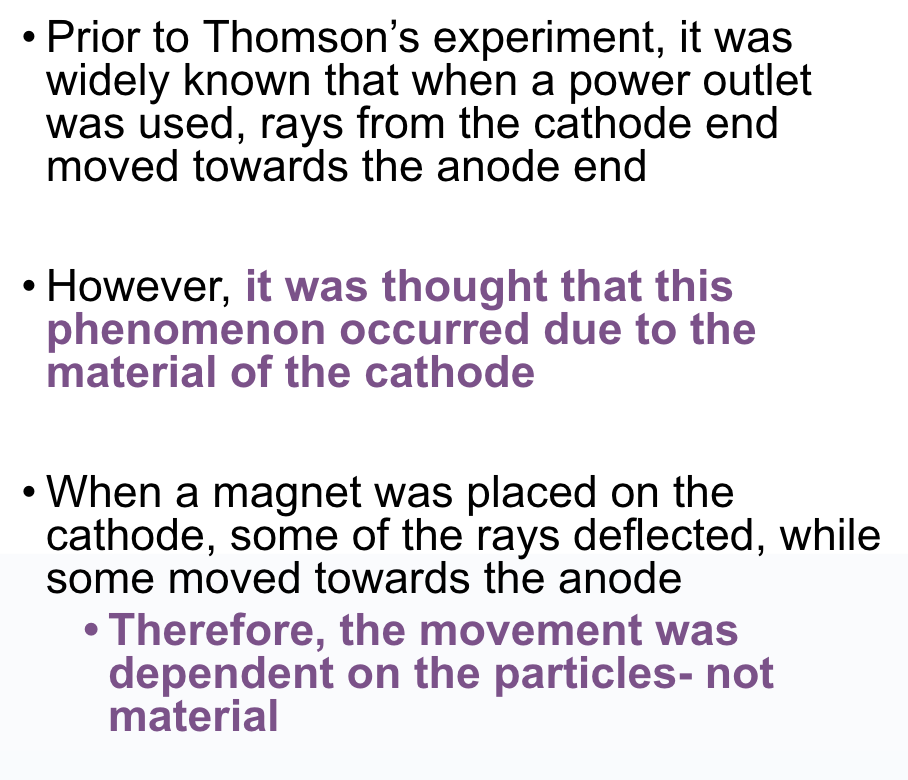
J.J. Thompson

Cathode Ray
Plum Pudding Model and Thompson’s Conclusions

Gold Foil Experiment

Rutherford’s Gold Foil Experiment

Rutherford’s Conclusions

Limitations with Rutherford’s Atomic Model

What are Quantum Numbers?
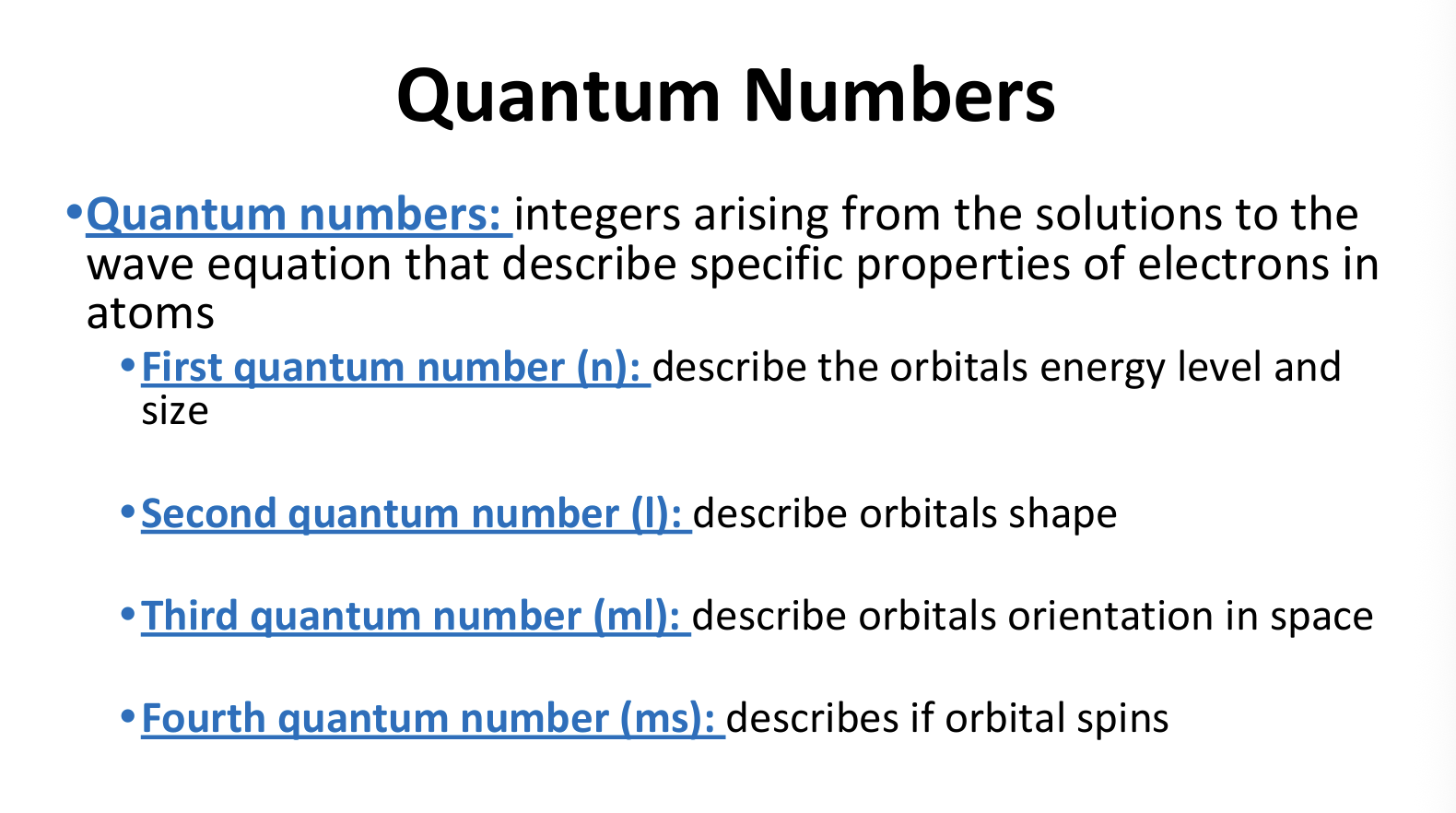
Values of l

Values of ml

Formula to Determine Max Number of Electrons
2N²
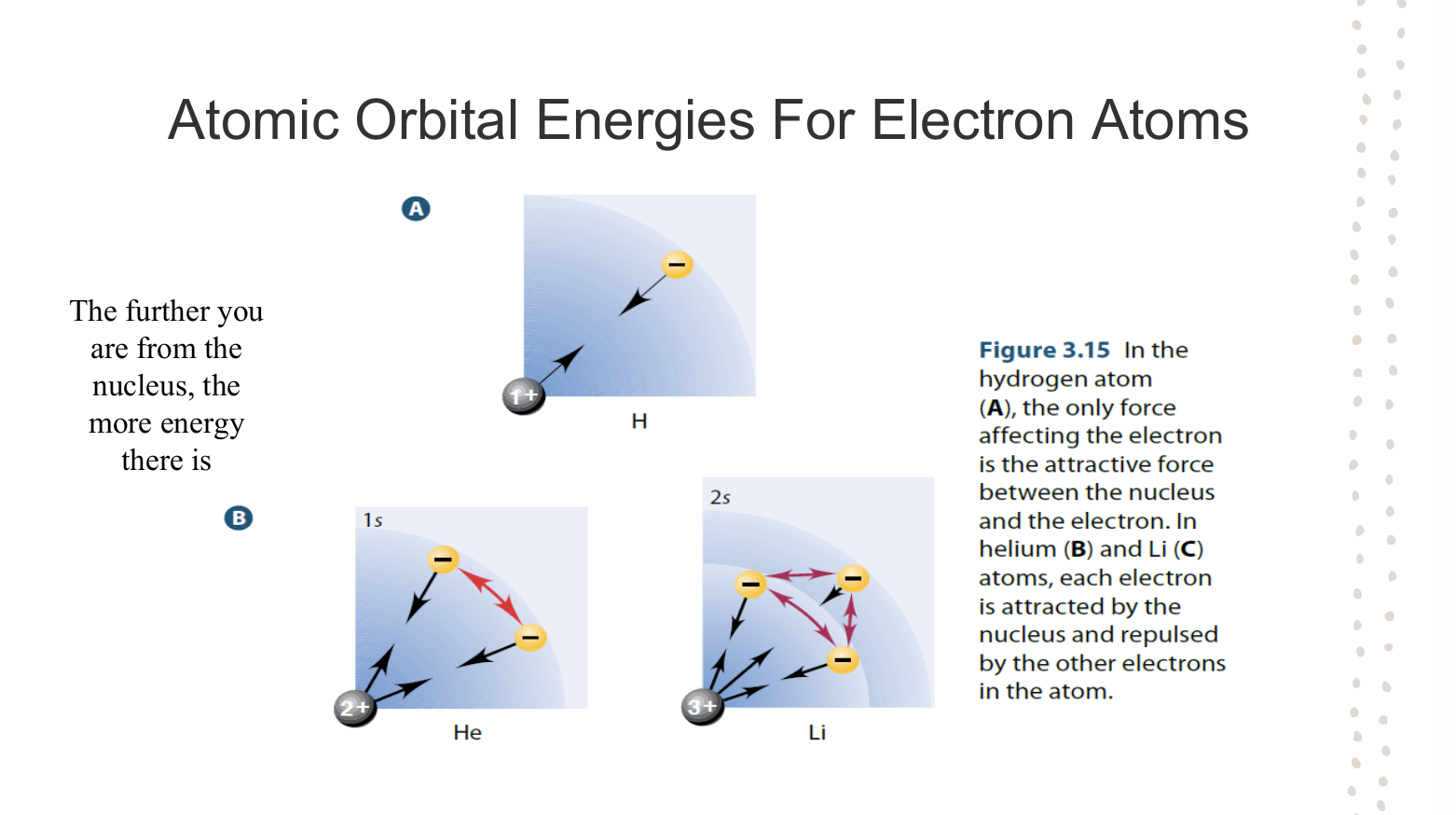
Presence of Electrons and Overall Energy Level

Energy Level Diagrams - Orbital Subheadings
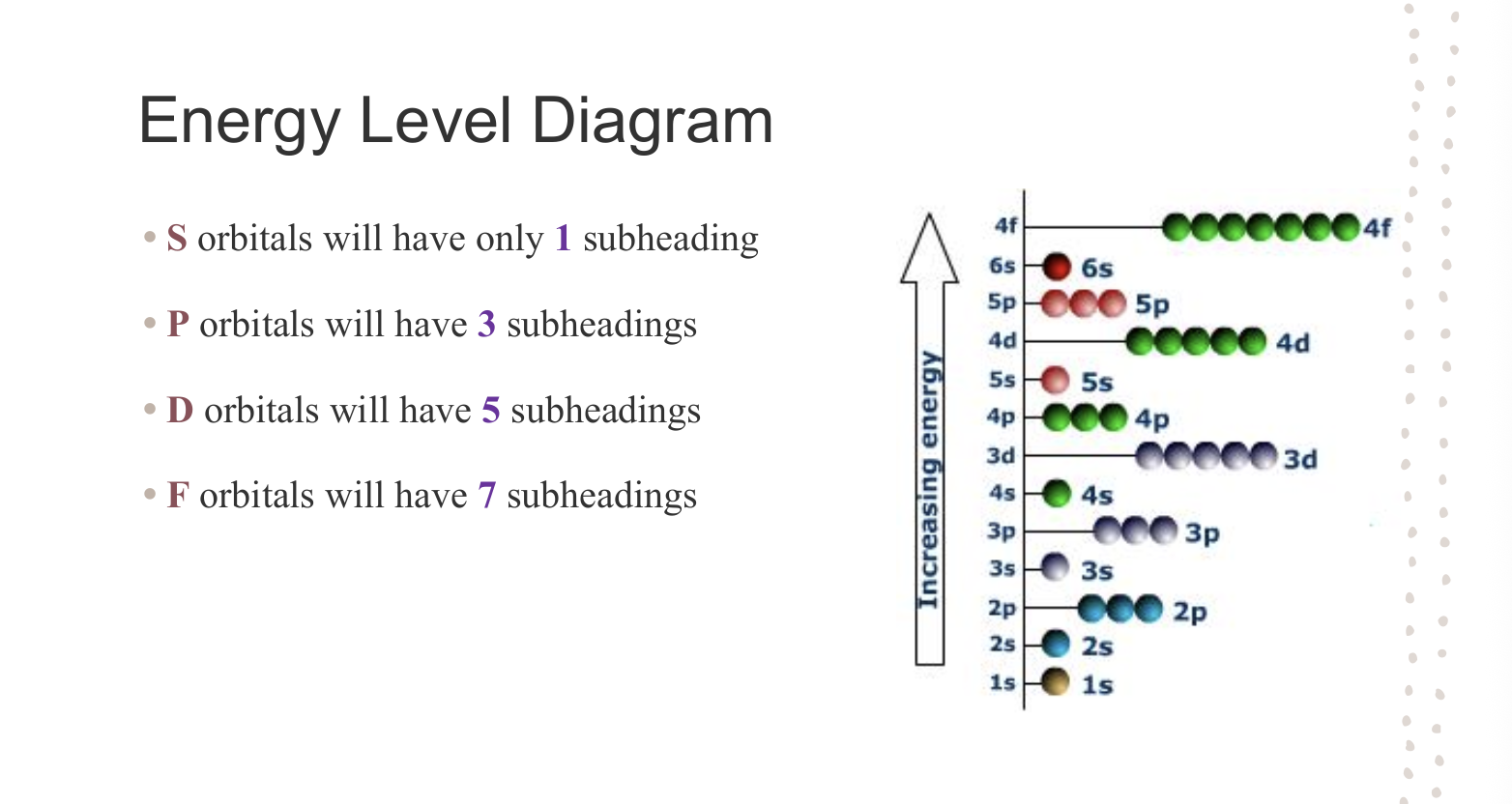
Rules/Principals of Energy Diagrams

Molecular Orbitals for S, P, D, F

Periodic Trends - Atomic Radii
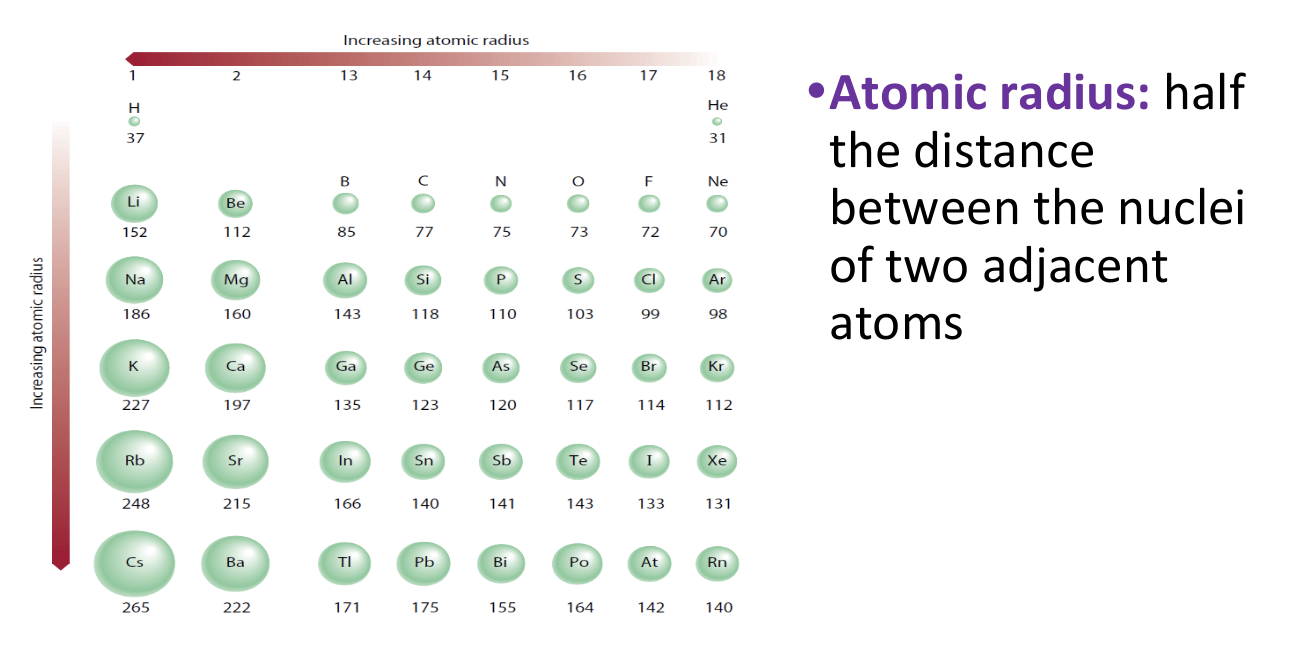
Factors Affecting Atomic Radius

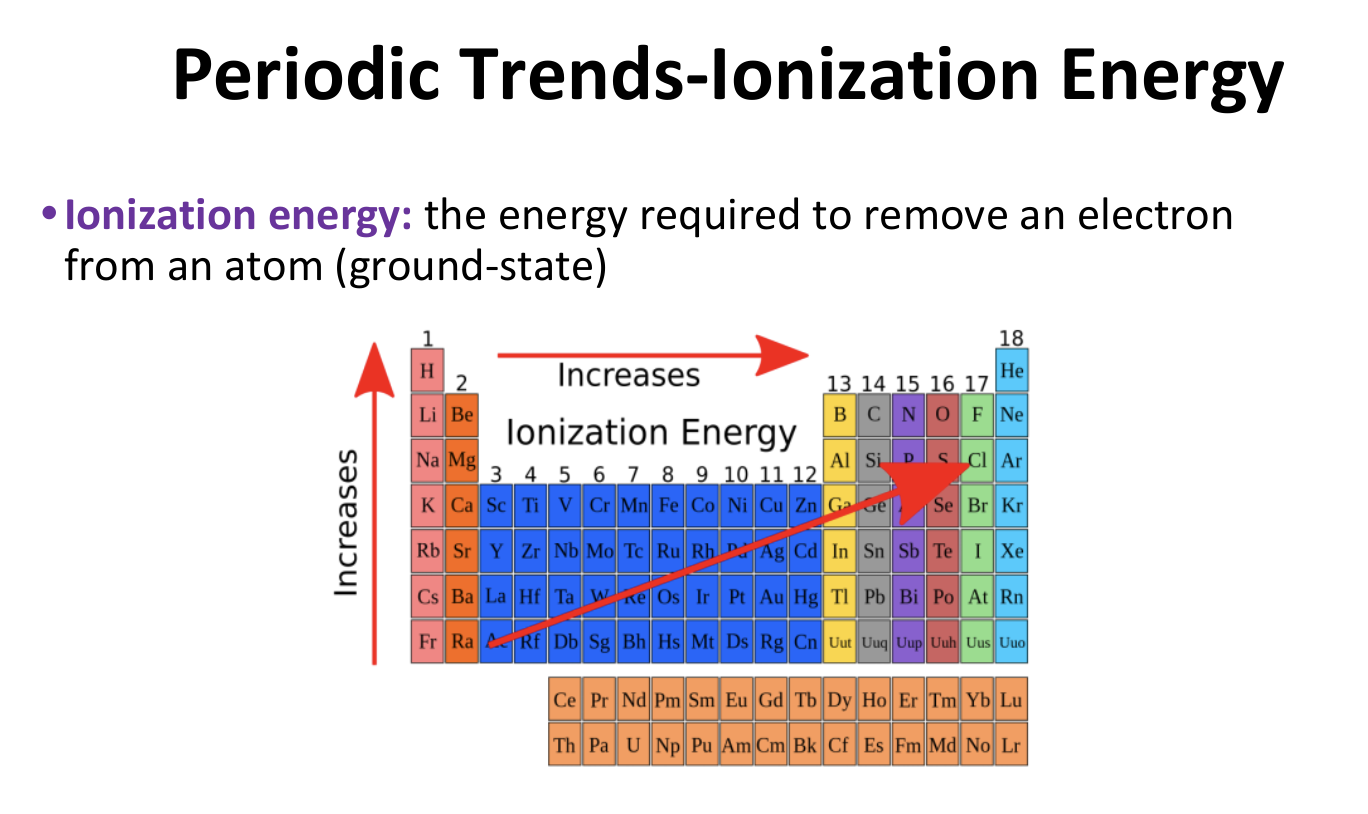
Periodic Trends - Ionization Energy
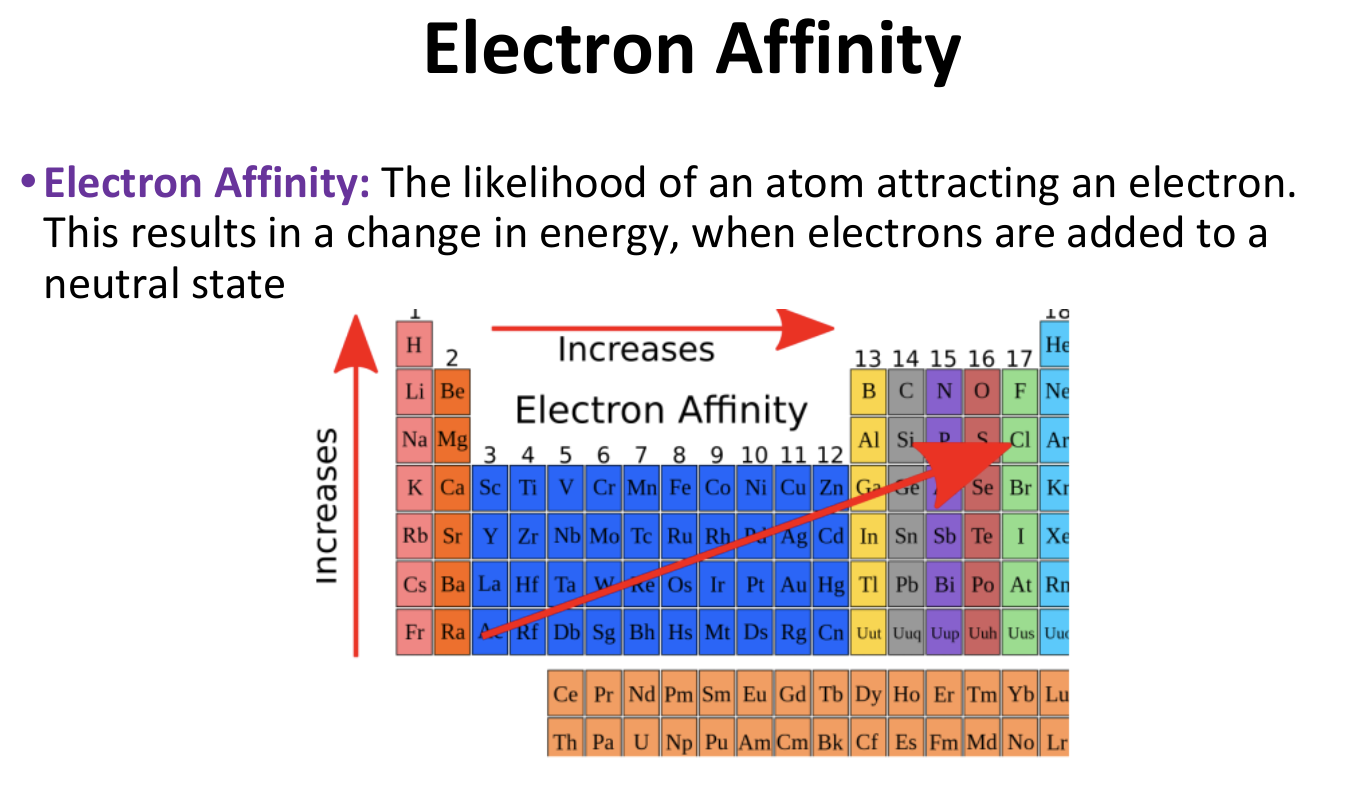
Periodic Trends - Electron Affinity