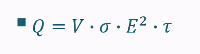5. Heat transfer (DONE)
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
38 Terms
Sensible heat
Heat you can feel
ΔT > 0
No phase change
Q = McΔT
J/kg*K
Thermal conductivity
High gamma: conductor (e.g. metals)
Low gamma: insulator
Foods can have a conductivity of 0.3-0.6 if they contain water.
Thermal diffusivity
α
Combination of 3 product properties
the measure of how quickly heat spreads through a material
Enthalpy
The energy content of:
A product with mass M
A flow with mass flow
Heating/cooling → we need as much heat Q as the material gains in enthalpy
± ΔQ = ± ΔH
Enthalpy is a “state variable” → independent of history'
T0 = reference temperature
Latent heat
Heat you cannot feel
ΔT = 0
Phase change
S ⇌ L ⇌ G
J/kg
Energy exchange in latent heat
Heat that is supplied to evaporate water into steam
Heat that is withdrawn to freeze a product
Heat that is supplied to sublimate ice
Sensible vs latent heat in a graph
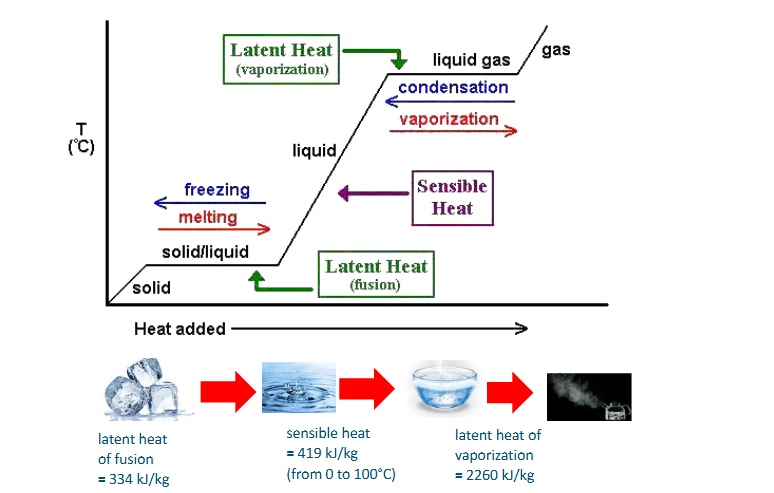
How to read a steam table
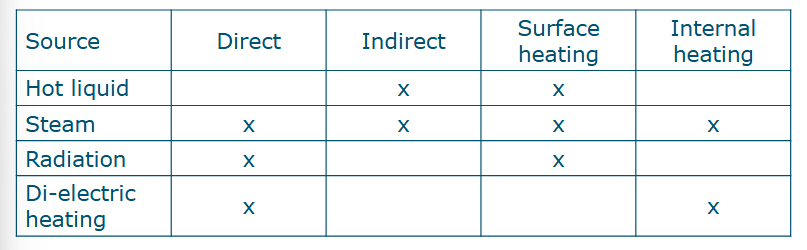
Different heating sources
Hot liquids
Water (T<100C)
Oil (T>100C)
Steam
Electricity
Heating mechanisms
Heat transfer by hot source
Hot water/hot oil
Steam
Heating by electricity
Radiation
Di-electric heating
Ohmic heating
Indirect/direct heating
Indirect: no physical contact of heating medium with product
Most common way to heat fluids, e.g. heating through a wall
Direct: physical contact of heating medium with product
Less commonly used: e.g. steadm injection
External heating three methods
Conduction
the process by which heat or electricity is directly transmitted through the material of a substance when there is a difference of temperature or of electrical potential between adjoining regions, without movement of the material.
Convection
the transfer of heat through the movement of fluids (liquids and gases) from a warmer to a cooler area
Radiation
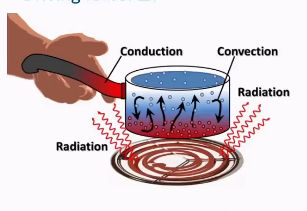
What are the heating mechanisms for hot liquid, steam, radiation and di-electric heating
Cooling
Definition
Removes heat from a product.
Refrigerant in cooling chamber:
Transfers heat from product → to outside environment.
Cooling Media
Water / ice water
Low-boiling-point media (e.g., ammonia, liquid nitrogen, isobutane)
Evaporation (L→G) requires latent heat, taken from the product → product cools.
After evaporation, refrigerant is compressed and re-liquefied → reused.
Steam
Fuel burning (coal, gas, oil)
→ convert chemical energy to heat energy
Indirect heating of water and conversion to steam
Steam collection in steam vessel
Distributed through piping system
How is steam created?
Created upon evaporation of water (as saturated steam)
Not diluted with air → saturated steam → use for heating
If diluted with air → moist air (not usable as hot source)
What is steam used for?
Used for heating
Use latent heat of condensation for indirect/direct heating of product
Industrial evaporation, re-use of vapor
Use released vapor (from product) as heating medium for next stage
Indirect vs direct steam heating
Indirect: steam heating
Heating of wall of vessel/pan
Direct infusion/injection of steam
Instant heating
Very efficient, very fast
Short heating time possible
Product is diluted with condensate.
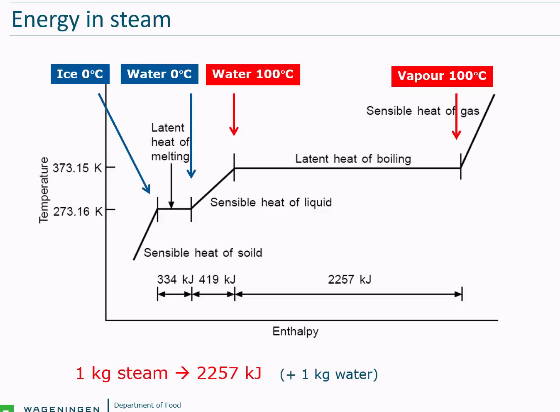
Energy in steam (sensible and latent heat in graph)
Mechanism of steam heating
Instant condensation
High rate of heat transfer
Fast T increase
Product slightly diluted
Steam temperature is regulated by pressure (more pressure = higher temperature of steam)
Flash pasteurization/sterilization
Direct heating
instant heating
Short holding time
Flash cooling of product by water evaporation
This provides better quality because:
There is less denaturation
Lower viscosity
Less nutrient loss
Conduction
Movement of heat: from hot to cold
Driving force: temperature difference
Applications: heating through a wall, heating through a semi-solid layer
Mechanism: direct transfer of molecular energy
Heat transfer by conduction

Relation of heat transfer (Q) to ΔT, wall thickness, thermal conductivity, heat transfer area.

What does the heat transfer coefficient tell us?
The rate at which heat moves in conduction
High HTC = heat moves very fast and very well
Low HTC = your wall is preventing heat transfer
Convection
Heat transfer in a fluid
Fluid molecules heated near surface wall (pan)
Molecules with high kinetic energy carried by the flow
Fluid takes up the heat and disperses it
Heat dispersed in the bulk is very fast
Convection is very fast compared to conduction
Free and forced convection
Free convection: due to density differences, caused by temperature differences (low h value)
Forced convection: mechanically enhanced (stirring, pump, air blowing) (higher h value)
Convection near a wall
Near wall: limited flow, so limited heat transfer
Limited heat transfer in the small layer close to interface
Close to wall: heat transfer by conduction
What does the convective heat transfer coefficient h depend on?
Fluid properties:
Viscosity
Heat capacity
Conductivity
Flow conditions:
Flow rate
Turbulent or laminar
Thickness of boundary layer
Geometry and dimensions
Nusselt number
Shows if there is more convective heat transfer or conductive heat transfer
𝑁𝑢=1: This signifies that heat transfer is dominated by conduction only, with no convection occurring.
𝑁𝑢>1: This indicates that convection is playing a role in heat transfer. The larger the value of 𝑁𝑢, the more dominant convection is.
𝑁𝑢>>1: A very large Nusselt number often suggests turbulent flow, which enhances heat transfer significant
Heat transfer by infrared radiation
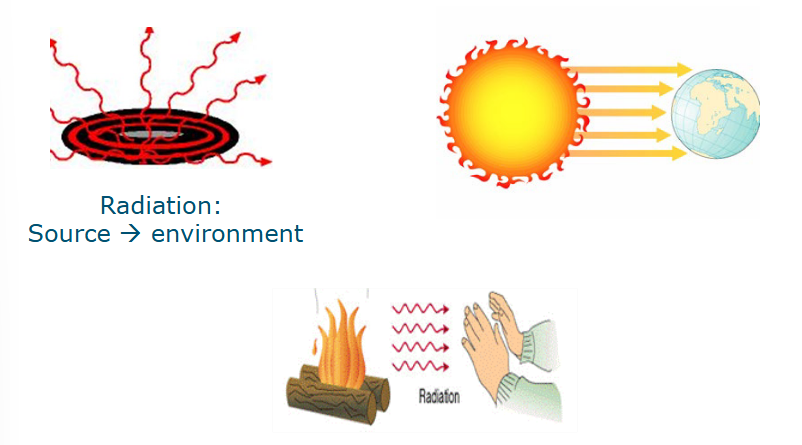
Infrared: Electromagnetic radiation
Emission of infrared radiation photons
Infrared light: wavelength of 700nm-1mm
Invisible to humans
Humans feel it as heat
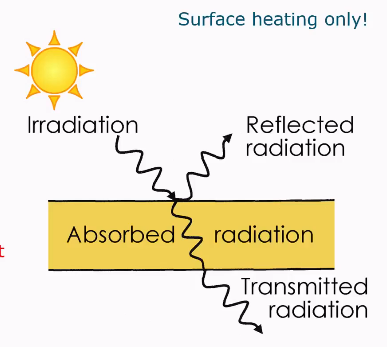
Infrared (halogen) radiation
Surface heating only
Electric radiation: expensive
Applications:
Heating: grill, oven
Drying: sun drying
Emission, absorption and reflection of radiant energy
Emission:
Any object with temperature above 0 K emits infrared radiation.Absorption:
Molecules absorb incoming photons → molecular vibrations increase → temperature rises.Reflection:
Some incoming radiation is bounced off the surface without being absorbed.
What does the Stefan Boltzman law show?
The radiation intensity and how it strongly depends on the temperature of the heating element.
ε = emissivity (a surface property of emitting material)
ε = 1 blackbody
ε = 0 mirror
σ = Stefan-Boltzman constant
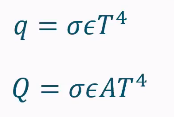
Di-electric heating
Electricity creates an alternating electric field
Electromagnetic radiation by:
Microwaves
Radiowaves
Mechanism Di-electric heating
Alternation electric field → alternating polarity
Many materials contain dipolar molecules
When the electric field reverses its direction millions to billions of times per seconds, the dipoles attempt to realign with it.
Dipoles cannot kepp up and this causes:
Internal friction
Collisions between molecules
Energy loss in the form of heat
Ohmic heating
Heating by electricity
Electric field creates voltage gradient
Alternating electrical current passes through the food
Ions in product move → heat generation
Requires electric conductivity of food