lec 26 - personalized treatment of disease (minden)
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
potential uses for stem cells in disease treatment
parkinsons
alzheimers
multiple sclerosis (MS)
amyotrophic lateral sclerosis (ALS)
spinal cord injury
diabetes
cardiomyopathy
liver failure
regenerative medicine
macular degeneration
cornea disorder
burns and skin disorders
most treatment involving stem cells are still in clinical trials
mostly HSCs
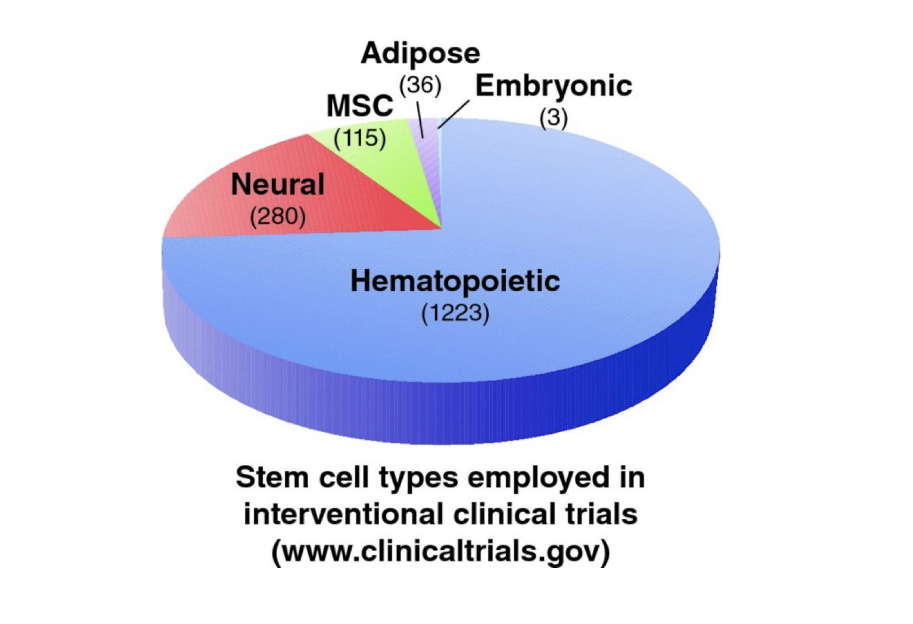
current status of stem cell treatments
majority of clinical trials at the current time involve the use of adult stem cells, esp. HSCs
many of these are designed for the treatment of non-blood diseases such as heart disease, diabetes, spinal cord injury, MS, epilepsy
many studies are still in early phases
some are farther along, such as those involving heart disease
in the case of heart disease, certain trials have shown that the procedures are safe and now they can be applied to larger groups of pts
only FDA approved stem cell treatments at this time are with adult HSCs
challenges and problems with stem cell treatments
while there are potential new stem cell treatments on the horizon, many challenges, leading to unrealized expectations and false promises
insufficient regulation
insufficient education of pts → leads to unrealistic hope
technical challenges with using stem cells
dealing with possible rejection by immune system
possible side effects including cancer
technical challenges: stem cell differentiation
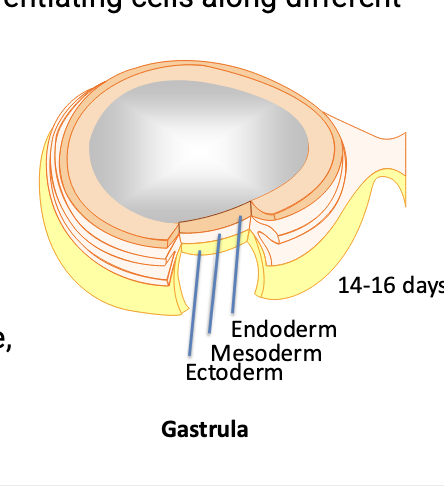
in order to use stem cells in disease treatment, important to start the differentiation process in vitro before introducing cells into patients
stem cell researchers need to develop methods for differentiating cells along different lineages
making cells differentiate into the cells of the ectoderm tissue (such as nerve cells) has been the most straightforward followed by mesoderm (such as heart and vascular cells)
endoderm tissue (such as lung and pancreas cells) = most difficult
implantation often has opposite pattern
for example, implanting cells into the pancreas can be easier than transplanting nerve tissue into the nervous system
road to the clinic
most studies go thru the following steps
basic research
studying the cells in the lab
using various models
including cell cultures and animals
preclinical research
testing the safety and efficiency of the new potential therapy using non-human models, including various animals
clinical trials
phase I → test the therapy on small group of people with the goal of assessing the safety of the treatment
phase II → give the therapy to a larger group of patients to test efficacy and to test safety in more depth
phase III → treatment is given to larger group of individuals to tests its efficacy; results are compared with other treatments/trials; only if this stage successful will the drug go on the market
phase IV → occurs after the drug is on the market to further asses risks and the best ways to use the treatment
stem cell medicine vs stem cell therapy
stem cell therapy = treating the patient with NEW stem cells
stem cell medicine = may involve pharmacologically activating the pts’ own adult stem cells such as in some cases of regenerative medicine
which type of stem cells to use for stem cell therapy
ES cells → pluripotent; many advantages
iPS cells → may in the future solve problems of tissue rejection
adult stem cells → countries in which Es cells are banned; have been helpful in paving the way for studying disease treatment with adult stem cells
some diseases and conditions for which stem cell therapy may hold promise
blood disorders, leukemia, aplastic anemia → HSC therapy
parkinson’s disease and stem cell therapy
one of the early diseases for which stem cell therapy has been studied
good candidate for stem cell therapy b/c a single cell type is involved
different approaches have been studied, involving different types of stem cells
good model for understanding stem cell treatment b/c different types of stem cells have been proposed or studied for treating the disease
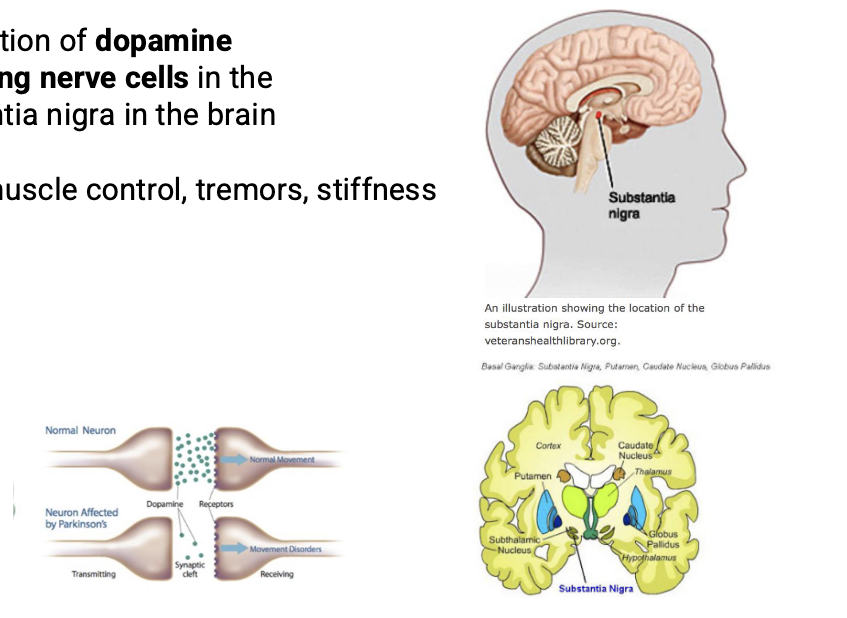
what is parkinson’s
degeneration of dopamine producing nerve cells in the substantia nigra
loss of muscle control, tremors, stiffness
treatment for parkinson’s
traditional methods rely on the use of L-dopa
L-dopa is converted to dopamine
can be effective for a while but regulating the dose is difficult
deep brain stimulation = newer treatment but does NOT improve all symptoms and results vary among different patients
newer methods that have been proposed involve the use of stem cells; some preclinical/clinical studies have been carried out
types of stem cells that hold promise for parkinson’s
fetal stem cells
MSCs
muse cells
ES cells
iPS cells
NSCs
parkinsons’s: fetal stem cells
some of the earliest studies
success was mixed
advantages
fetal cells have strong capacity for proliferation and differentiation
fetal cells often do NOT induce an immune reaction
disadvantages
ethical concerns, moratoriums (temp. ban), use of aborted fetuses, informed consent issues
availability of fetal stem cells; can be scarce, even within the fetus
results have been mixed, only some studies have been succesful
tissue rejection
contamination with other neurons, including serotonin neurons
parkinsons’s: MSCs
MSCs can differentiate into several cell types
several protocols have been developed to differentiate MSCs into dopamine neurons
these differentiated MSCs have been transplanted into rat models of PD
results = relief of some symptoms
some researches also interested in using MUSE cells (subset of MSCs) to treat PD due to several advantages these cells may have over MSCs
PD: iPS cells
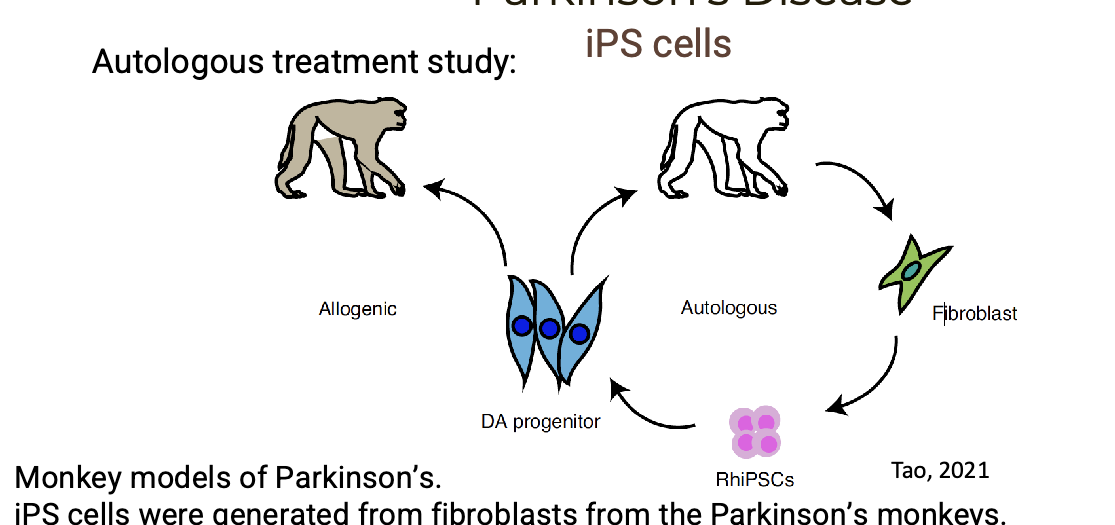
iPS cells were generated from fibroblasts from the PD monkeys → cells were differentiated into DA neurons
neurons were transplanted ack into the monkeys (autologous) or into other monkeys (allogenic)
autologous recipient showed improved movement and decreased signs of depression
PD: iPS/NSCs
clinical trial that involved the use of a type of NSCs
NSCs that were developed from iPS cells that originally came from the patients peripheral blood mononuclear cells
transplanted into the brains of the same pts by injection (autologous treatment)
pts will be followed for at least 3 years
some clinical trials that have also been started involve ES cell line
challenges for using stem cell studies in PD
getting stem cells to become functioning neurons
methods for delivering the stem cells to the right targets
learning how to get the stem cells to integrate into the brain
importance of collaborative research
alzheimers disease
more complex than PD b/c multiple cell types are most likely involved
most common cause of dementia
complex disease that affects the nerves of many parts of the brain
makes effective treatment challenging
precise cause of the disease = unknown
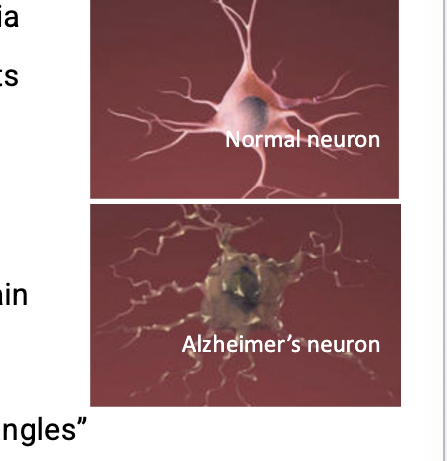
people with alzheimers have an abnormal build up of certain proteins in the brain
include amyloid beta which clumps together to form “plaques” and tau which gets twisted into protein “tangles”
one theory is that the plaque prevents nerve cells from communicating properly while tangles make it difficult for the cells to get the nutrients they need
role for these plaques = unclear as recent studies show that they might NOT be as important as previously thought in the disease
more about alzheimers
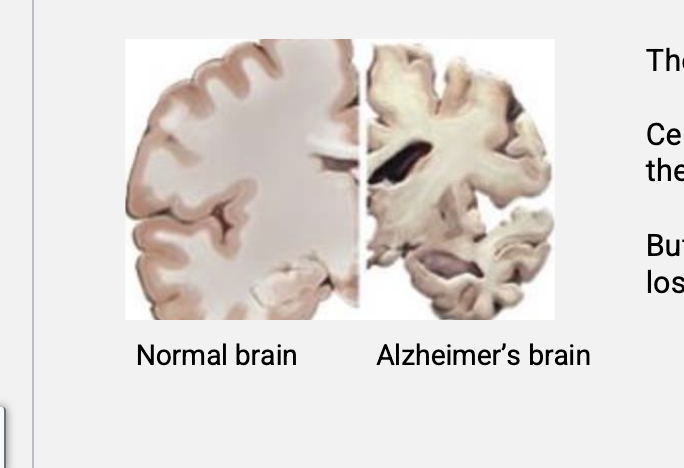
neurodegenerative disease
over time, certain nerves die
NO cure for alzheimers
certain drugs can temporarily help with some of the symptoms but there are NO drugs that prevent or delay the loss of neurons
stem cells and alzheimers
stem cell therapy = active area of research with much of the work being done in animal models
challenges
many parts of the brain are affected so stem cells would have to travel to multiple areas of the brain
stem cells would have to differentiate into many types of neurons
new neurons would have to integrate and make the right connections
even if the brain can integrate new neurons, can they do so after the alzheimers has started?
would the new stem cells be damaged by the tau protein that already in the brain of alzheimers?
alzheimers: iPS cells
in vitro studies using iPS cells
some but NOT all cases of alzheimers may have a genetic component (familial)
researchers have developed iPS cells from skin cells of patients thought to have familial alzheimers
these iPS cells were differentiated into neurons and grown in culture
in some cases these lab-grown neurons released beta amyloid protein
this can give scientists a tool for studying the disease in vitro and testing drugs
alzheimers: HPCS cells (subset of HSCs)
hematopoietic stem cell progenitor cells (HPCS) = slightly more differentiated form of HSCs
healthy HSCs and HSPCs were transplated into the brain of an AD mouse model
symptoms of AD were reversed, including memory and cognition problems; neuroinflammation was and beta-amyloid buildup was reduced
multiple sclerosis (MS)
MS affects the CNS
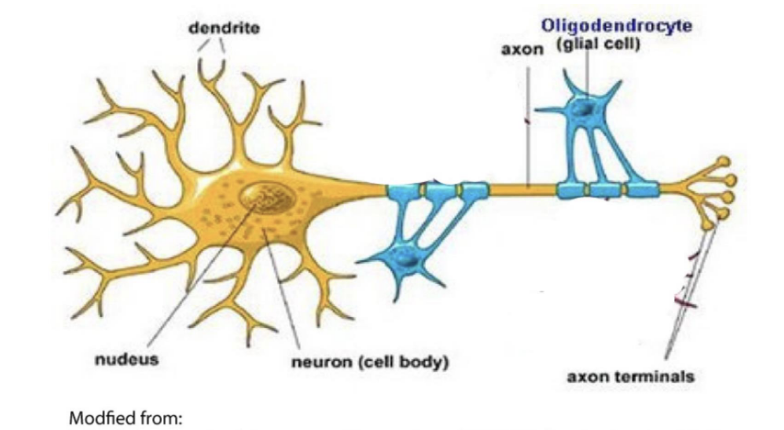
part of family of diseases the involve the lack of myelin
myelin insulates the axons of nerves
oligodendrocytes = cells that produce myelin
patients’ immune system attacks the myelin sheaths surrounding nerve fibers → leads to breakdown in the transmission of nerve signals
symptoms can range from numbness to blindness and paralysis
current treatments often involve immunosuppression
treatment = effective but usually temporary
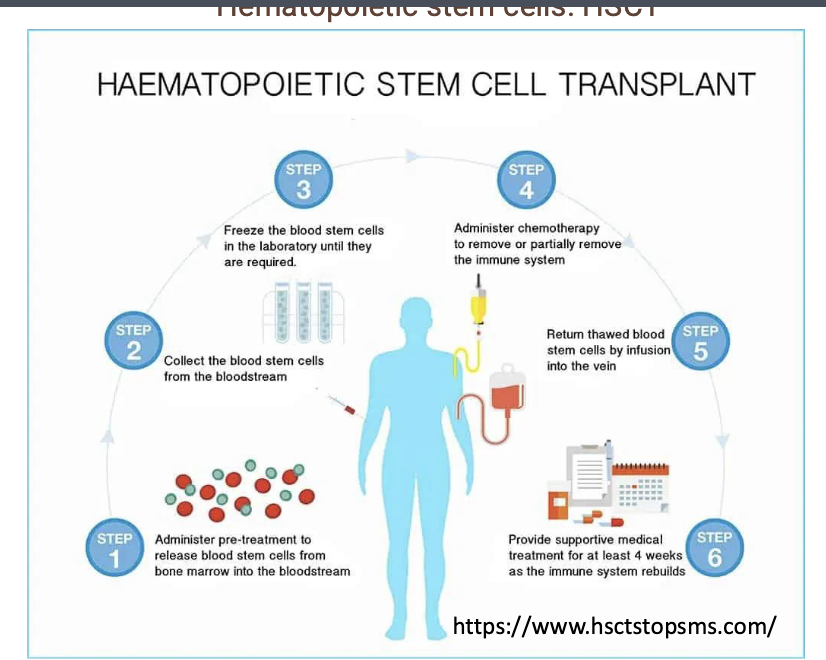
MS: hematopoietic stem cell transplant (HSCT)
autologous hematopoietic stem cell transplant (HSCT) clinical trial for MS
in this trial, patients bone marrow stem cells (HSCs) are harvested
patients immune cells are intentionally destroyed
patients are then injected with their own HSCs
re-injection apparently ‘resets’ the bodies immune system
high percentage of patients who did NOT respond well to traditional therapies had significant improvement of symptoms
though promising, NO approved HSCT treatments yet in the US but some patients are receiving this treatment as part of ongoing clinical trials
MS: HSCT process
administer pre-treatment to release blood stem cells from bone marrow into bloodstream
collect the blood stem cells from the bloodstream
freeze the blood stem cells in the laboratory until they are required
administer chemotherapy to remove or partially remove the immune system
return thawed blood stem cells by infusion into the vein
provide supportive medical treatment for at least 4 weeks as the immune system rebuilds
MS: other possible stem cell sources for treating MS
adult MSCs are being tested in clinical trials
patients MSCs are isolated from bone marrow or blood and multiplied then re-introduced into patients in higher numbers
iPS cells
take the patients skin cells and make them into precursors of oligodendrocytes
goal would be to re-introduce these into patients to restore the damaged oligodendrocytes
MS: MSCs
iPS cells can lead to tissue regeneration in damaged tissue but another additional reason that MSCs may be beneficial for MS is that they have immunosuppressive activity
mechanism by which MSCs have immunosuppressive properties is NOT entirely understood but seems to involve a combination of nitric oxide (NO) production and chemokine secretion
MSCs produce NO
NO known to suppress T cell proliferation → suppress the immune system

good sources of adult stem cells
scientists who work with adult stem cells are always interested in identifying the tissues that are the best possible source of adult stem cells
adipose derived stem cells (ASCs)
ASCs
origin of ASCs = unknown
methods for obtaining and using ASCs
fat tissue is collected
ASCs isolated from fat
ASCs expanded: self replicated in lab to produce more cells
ASCs guided to differentiate into different cell types
osteogenic
chondrogenic
adipogenic
potential uses for ASCs
regenerative medicine
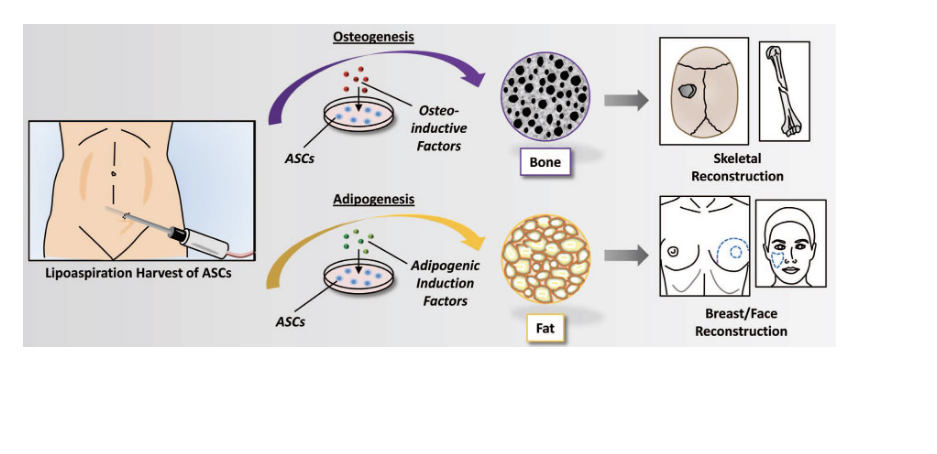
lipoaspiration to harvest ASCs
2 pathways
osteogenesis (bone formation)
ASCs are exposed to osteo-inductive factors → differentiate into bone cells → used for skeletal reconstruction
adipogenesis (fat formation)
ASCs are exposed to adipogenic induction factors → differentiate into fat cells → breast/face reconstruction