Chapter 21 -Carboxylic Acid Derivatives
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
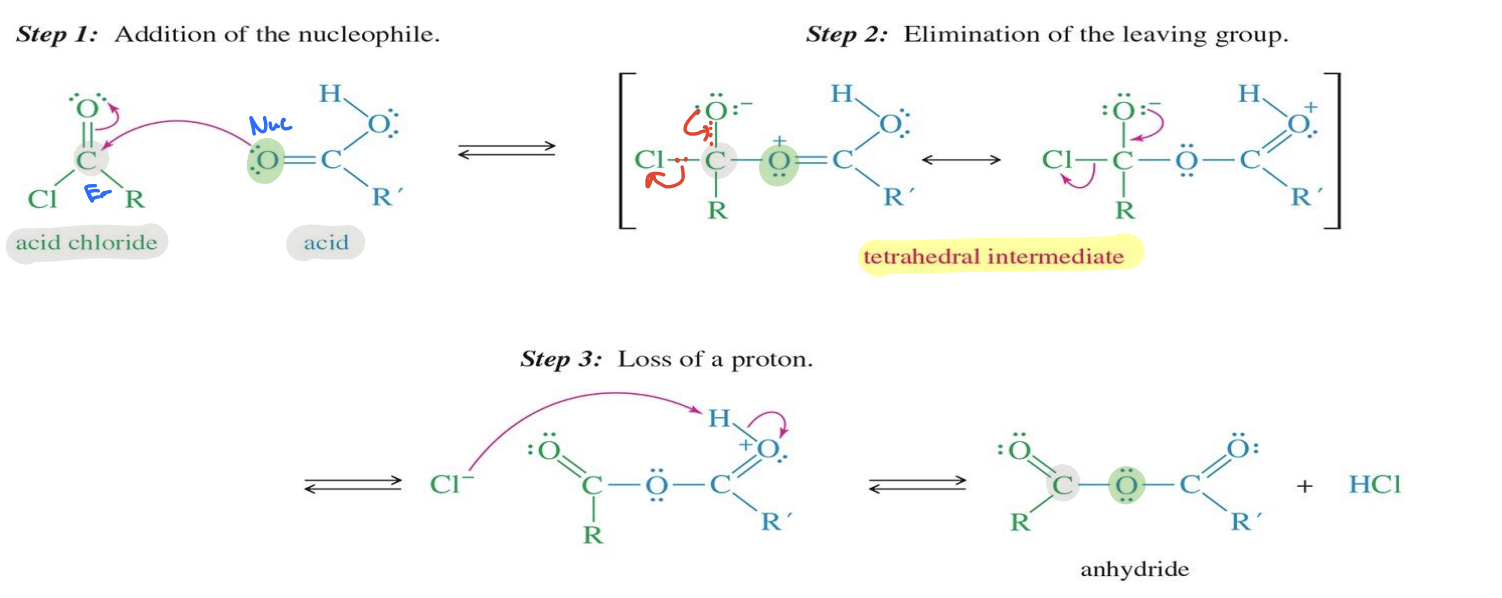
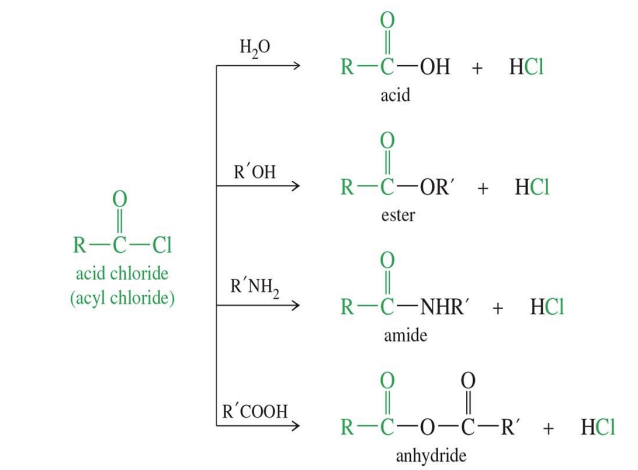
Acid Chloride to anhydride
Starting
Acid Chloride ( Cl - C (=O) - R)
Reagent
carboxylic Acid
End product Anhydride ( R - CO - O - CO - R)
Mechanism
Addition of the reagent : the Oxygen from the carboxylic attacks to acid chloride → the double bond oxy of the acid chloride becomes a singe bond
A Tetrahedral intermediate forms
Chloride (Cl⁻) leaves, restoring the C=O double bond
The Cl removes a proton (H⁺) → forms the anhydride product
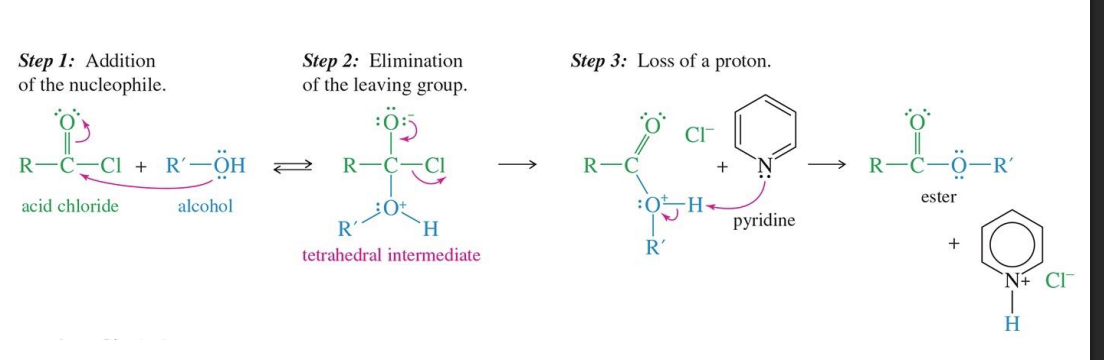
Acid Chloride to Ester
Starting
Acid Chloride
Reagents
Alcohol
Pyridine
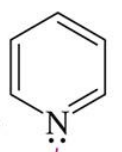
Mechanism
the alcohol attacks the acid chloride forming tetrahedral. ( the double bond with O of acid chloride becomes a single bond)
the Cl Leaves causing the double bond with oxygen to be restored
The Pyridine comes in and take a hydrogen from R - OH
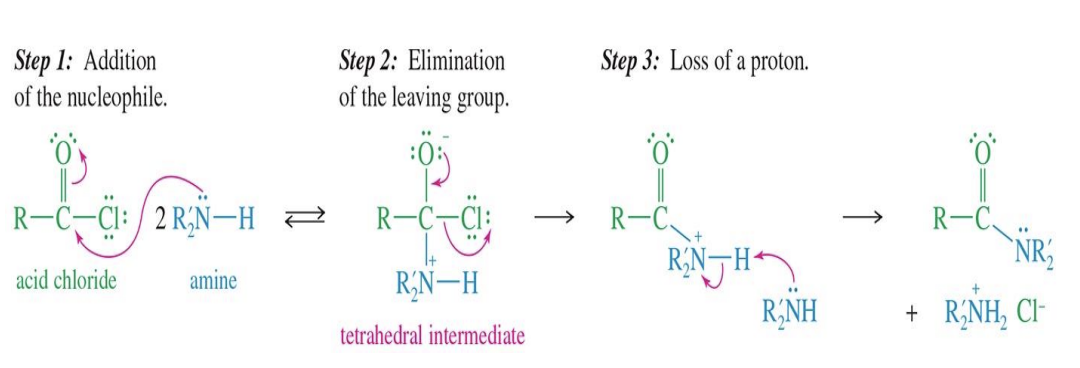
Acid chloride to Amide
Starting
Acid chloride (R-C(=O)-Cl)
Reagent
Amine (R₂N-H) — can be ammonia, primary amine (1°), or secondary amine (2°)
Mechanism
Step 1: Addition of nucleophile
The amine's nitrogen attacks the carbonyl carbon
Forms tetrahedral intermediate
Step 2:
Chloride ion (Cl⁻) leaves
Restores the C=O double bond
Step 3: Loss of a proton
Another Amine removes a proton from the nitrogen
Forms the amide product
Product types:
Ammonia (NH₃) → primary amide (1°)
Primary amine (RNH₂) → secondary amide (2°)
Secondary amine (R₂NH) → tertiary amide (3°)
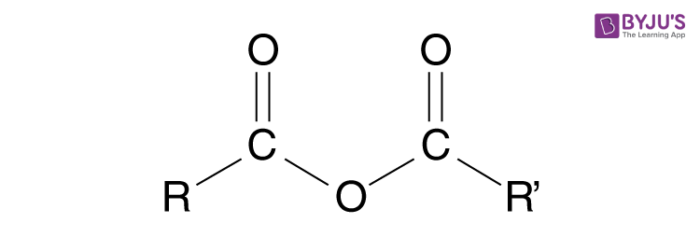
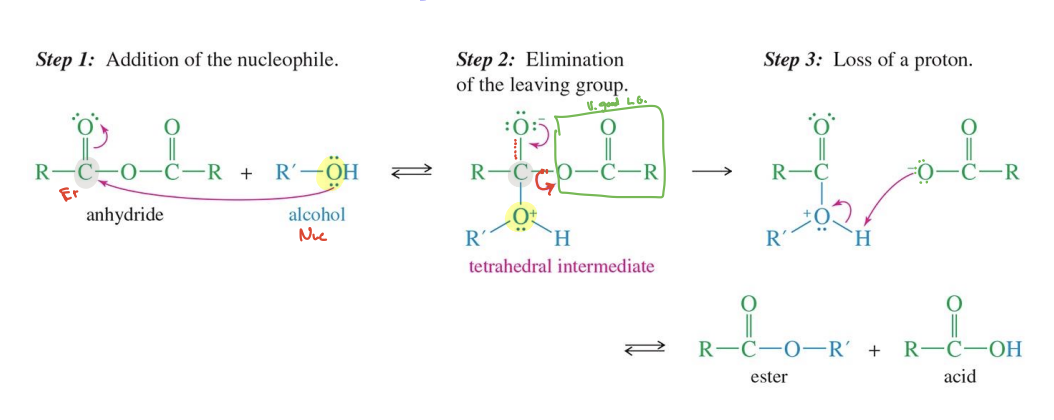
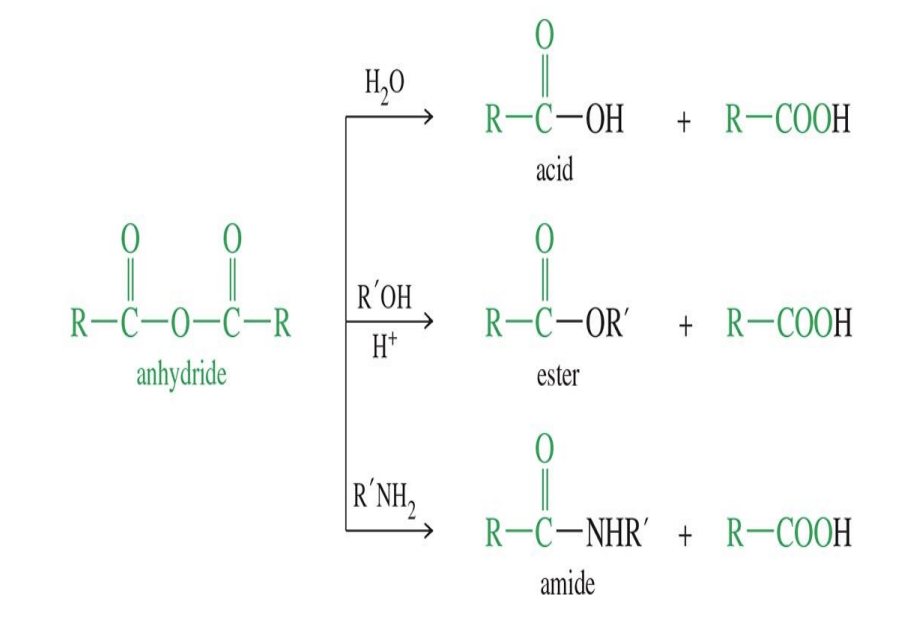
Anhydride to Ester
Starting:
Anhydride ( R - CO - O - CO - R)
Reagent:
Alcohol ( R-OH)
Mechanism
Alcohol Attacks one carbonyl group of the anhydride forming a tetrahedral intermediate
The other acid salt (carboxylate salt) ( O - C=O - R) acts a leaving group
The acid group that left takes a hydrogen from the R - OH
End product:
Ester
Acid
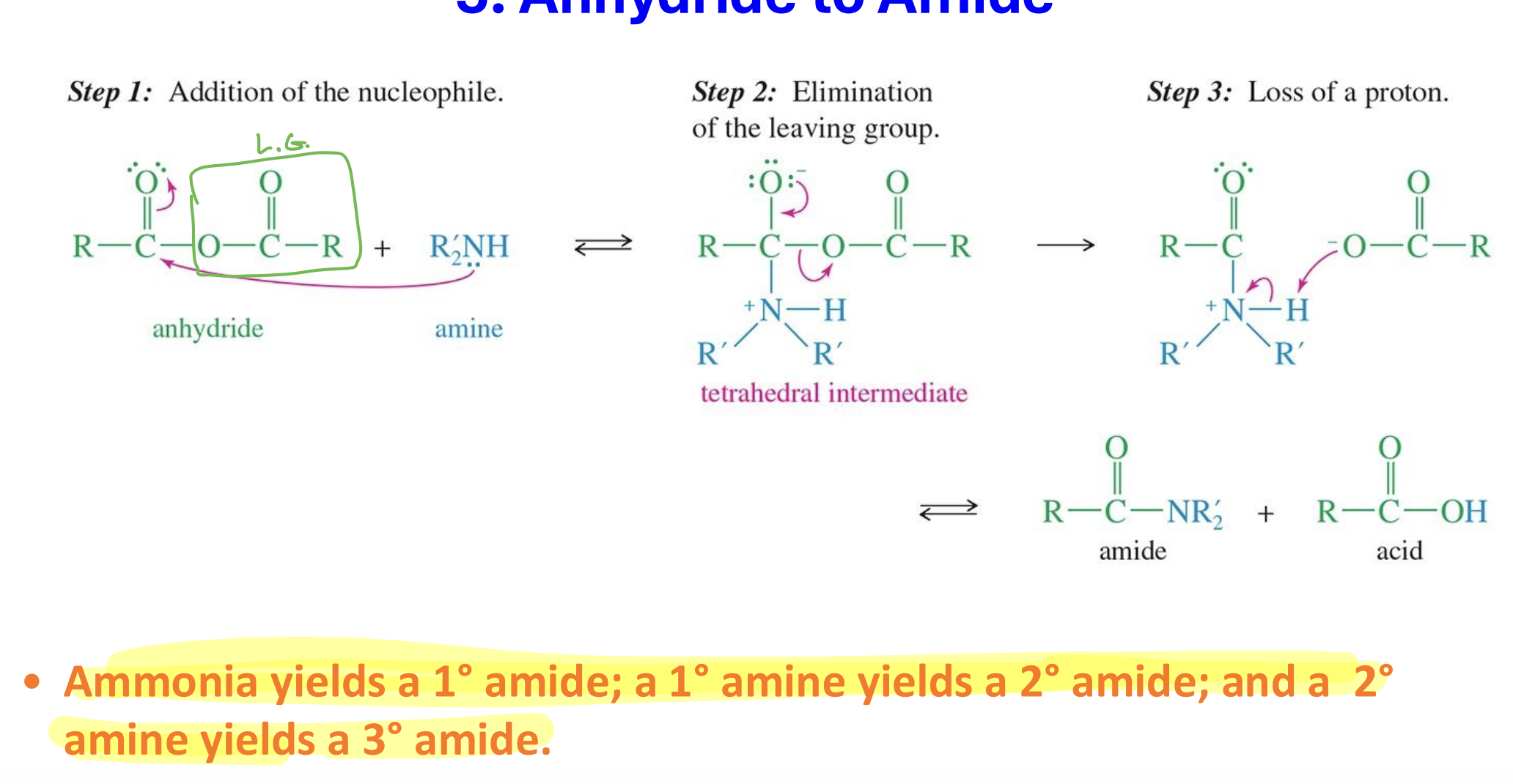
Anhydride to amide
Starting
Anhydride
Reagent:
Amine ( can be can be ammonia, primary amine (1°), or secondary amine (2°)
Mechanism
the amine attacks the carbon forming a tetrahedral
the acid group ( O - C=O - R) acts a leaving group
The same acid takes the Hydrogen from the amine
End product
Amide
Acid
Product types:
Ammonia (NH₃) → primary amide (1°)
Primary amine (RNH₂) → secondary amide (2°)
Secondary amine (R₂NH) → tertiary amide (3°)
Ester to amide ( Ammonolysis)
Starting
ester ( R - CO - OR)
Reagent :
NH3 Or 1° Amine (R - NH2)
MUST BE AMONIA OR 1° AMINE
Mechanism
the amine attacks the carbon of the ester
a tetrahedral intermediate is formed
the OR acts asa leaving group and breaks the bond with carbon
The same OR takes a hydrogen from the Nitrogen in amine
End Product
Amide
Alcohol
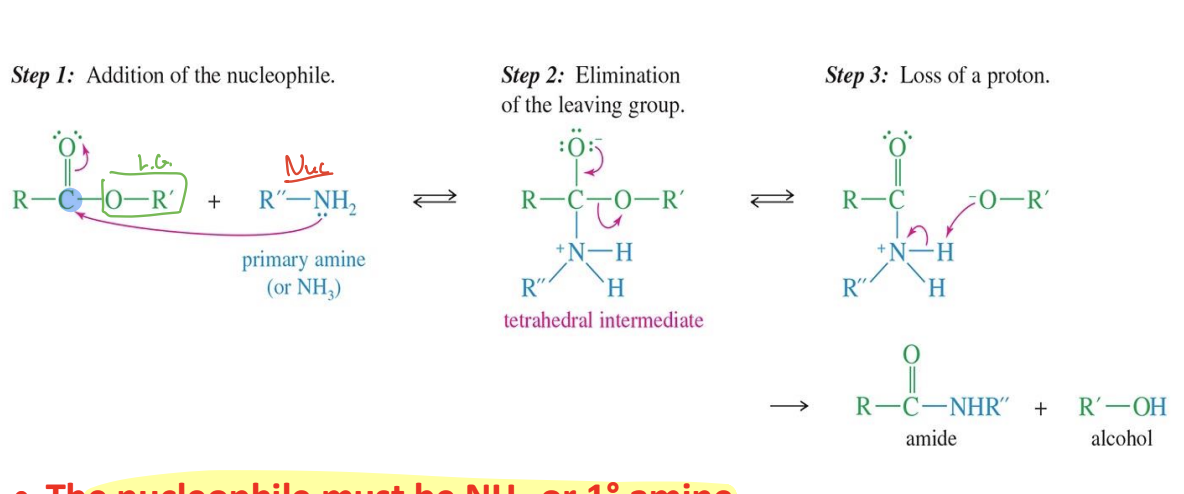
Hydrolysis of acid chlorides and Anhydrides
Starting
Acid chloride or Anhydrides
ReagentL
Water (H2O)
Mechanics
water comes in attacks the carbon from acid chloride adding H- O -H
the Chloride (Cl) leaves
Another water comes in a removed a hydrogen from H-O-H
End product
carboxylic acid
H3O+
With Anhydrides → 2 carboxylic acid
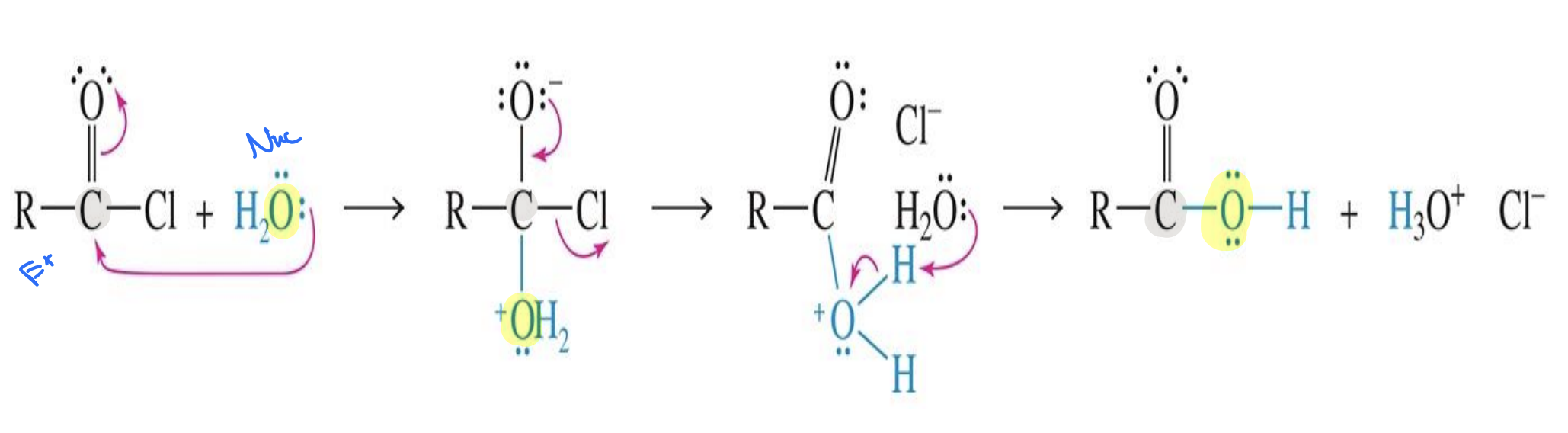
Hydrolysis of Amides
Starting :
Amide → R–C(=O)–NHR′
Reagent:
H3 O+ ( or strong acid H2SO4)
H2O
Mechanism
Part 1:
the Amide Oxygen attacks the H+ (acid) taking a hydrogen making (OH)
water comes in and attacks the carbon of the amides cause the double bond with oxygen and carbon to become single bonded
Another water comes in and takes a hydrogen from the H-O-H
Part 2:
Another Acid (H+) comes in and the Nitrogen (NH2) attacks it taking a Hydrogen making (NH3)
The NH3 Leaves restoring the double bond with carbon and oxygen
the NH3 takes the Hydrogen from the O
End Product:
carboxylic acid
NH4
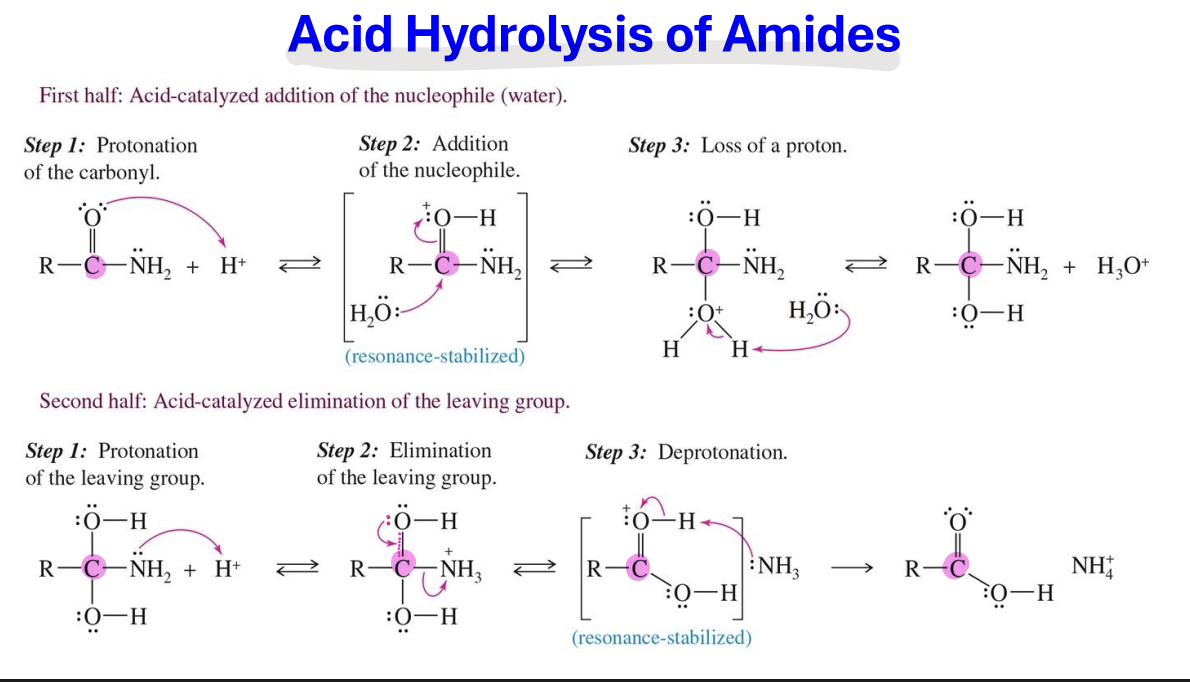
Hydrolysis of Nitriles
Starting:
Nitriles ( R–C≡N)
Reagent :
OH , H2O → basic
H+ , H2O → Acidic
End product
under acidic condition = Carboxylic acid
Basic condition = Carboxylate ion
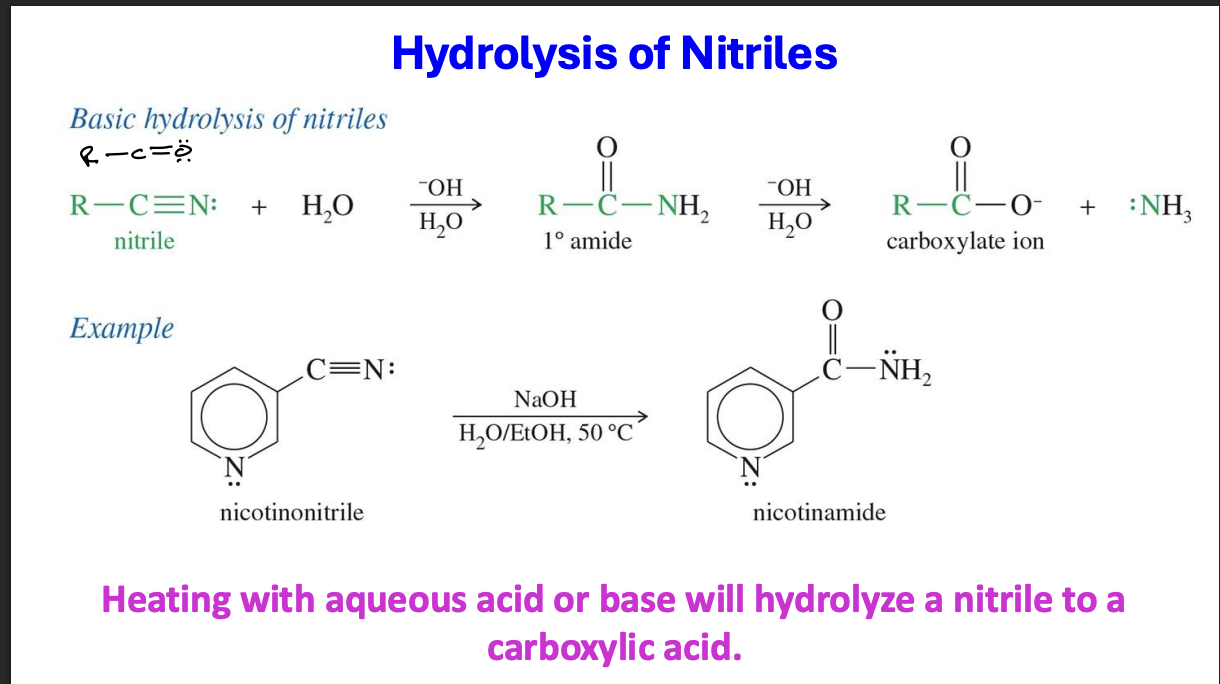
Reduction of Esters, carboxylic acid, acid halide to 1 Alcohols
Starting
Ester: R-C(=O)-O-R
Carboxylic Acid : R-C(=O)-OH
Acid chlorides R-C(=O)-Cl
Reagent:
LiALH4
LAH
H3O+
Mechanism
the Carbon of the starting reagent attacks the LiAlh4 taking a hydrogen from the reagent , the double with Oxygen becomes singular
A tetrahedral intermediate is formed R - C- O- OR
The OR leaves and he double bond is restored → forming an aldehyde
Another LiALH4 comes in and the carbon of the aldehyde attacks it taking a Hydrogen breaking the double bond
Acid comes in and the Oxygen takes a hydrogen forming a primary alcohol
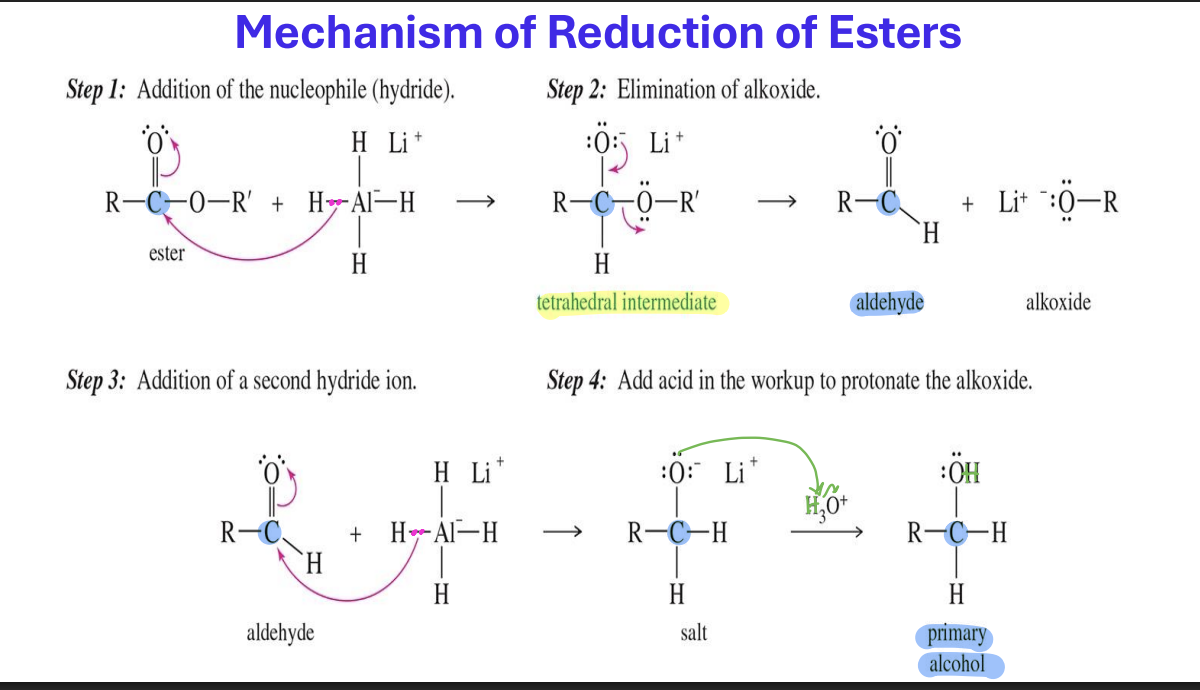
Reduction of Acyl Halides to Aldehydes
Starting :
Acyl Halides ( R - C ( = O) - X)
Reagent
LiALH ( O- t -BU)3
End product
aldehyde
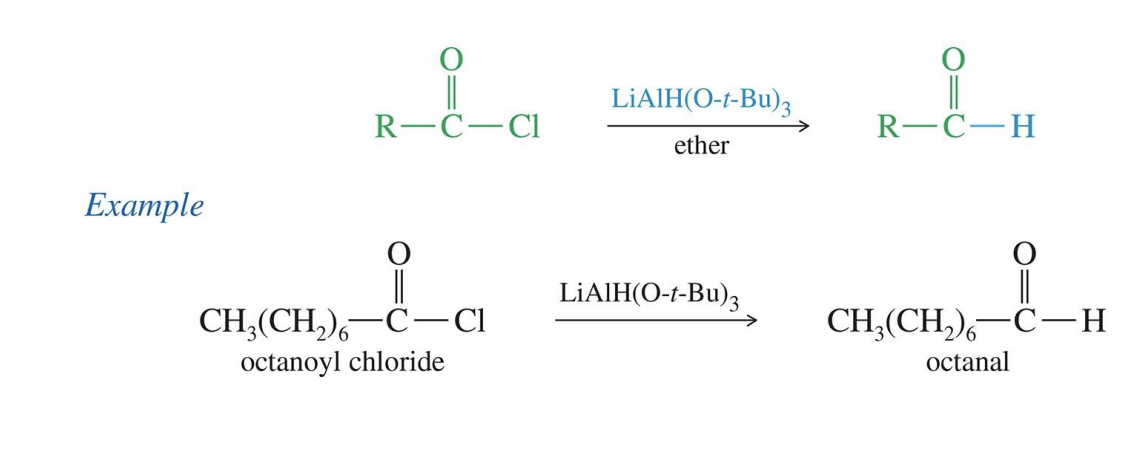
Reduction to aldehydes with DIABL
Starting
Esters R - C (=O) - OR
Reagent
DIABL or DIBAL -H
(i-Bu)2ALH
End product
aldehydes
Reduction of amide to an amine
Starting:
Amide ( R - C ( = O ) - NH2
Reagent:
LiALH4
H2O
End product
amine
R - CH2 - NH2
R - CH2 - NH - R
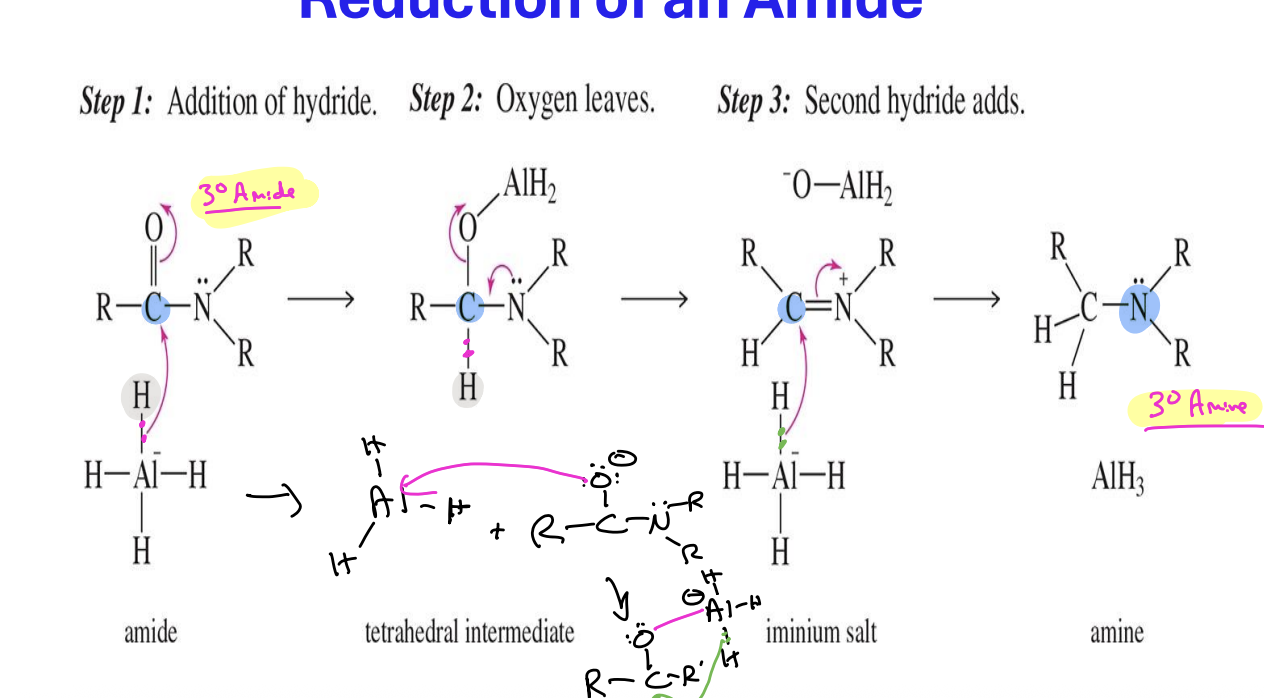
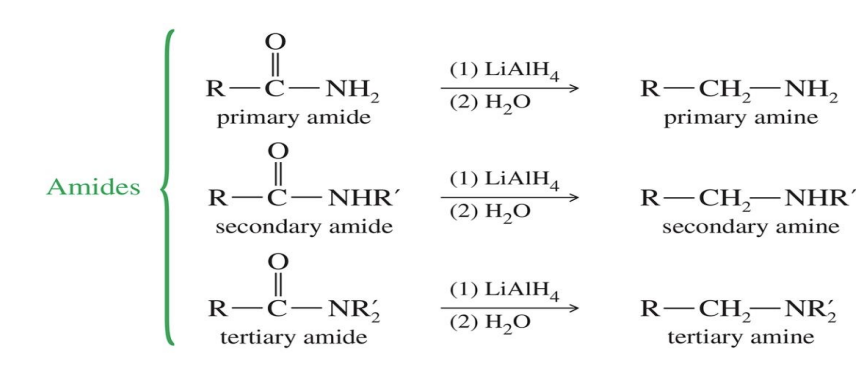
Reduction of Nitriles to primary Amines
Starting:
Nitriles R–C≡N
Reagent:
LAH or LiALH4
H2O
Mechanism
One of the hydride ions (H⁻) from LiAlH₄ attacks the electrophilic carbon of the nitrile triple bond. This adds hydrogen to the carbon.
A second hydride attacks. Now you have two H atoms added to the carbon
When the water comes in it give two more hydrogen to nitrogen making the Al leave
End product primary amines
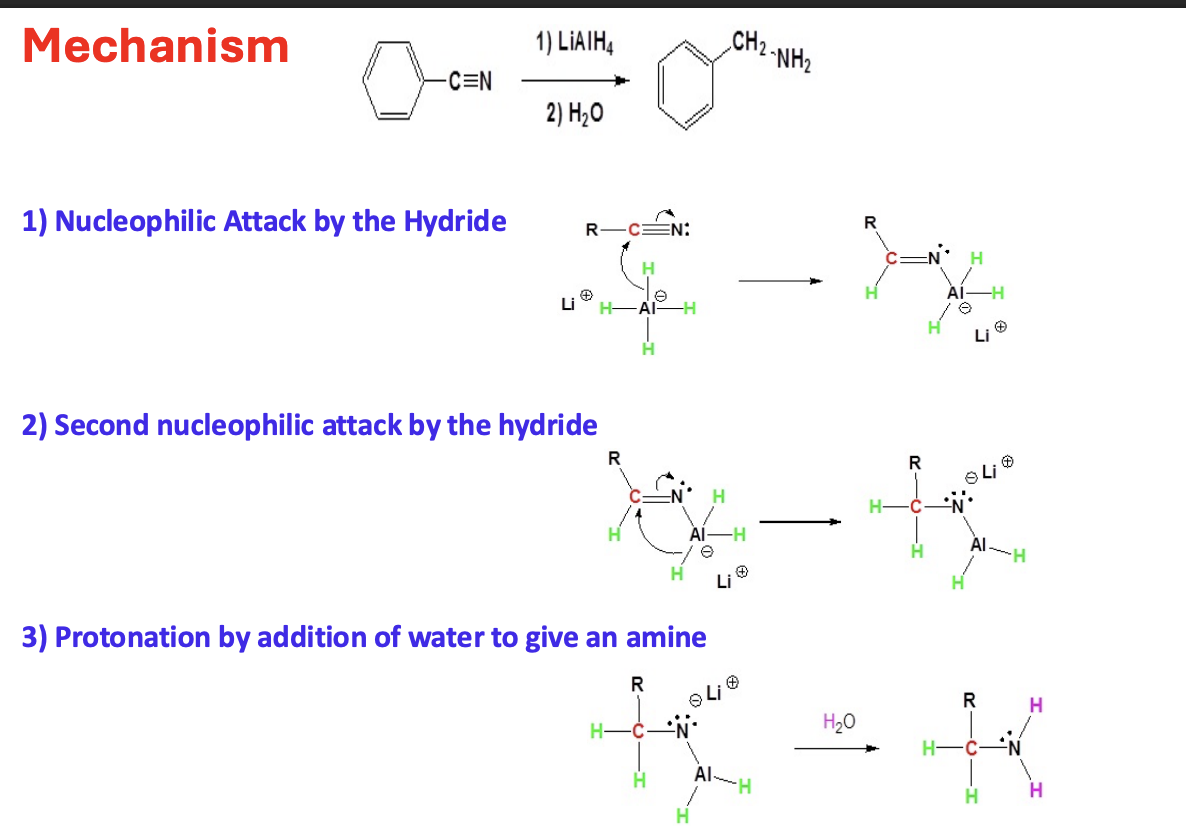
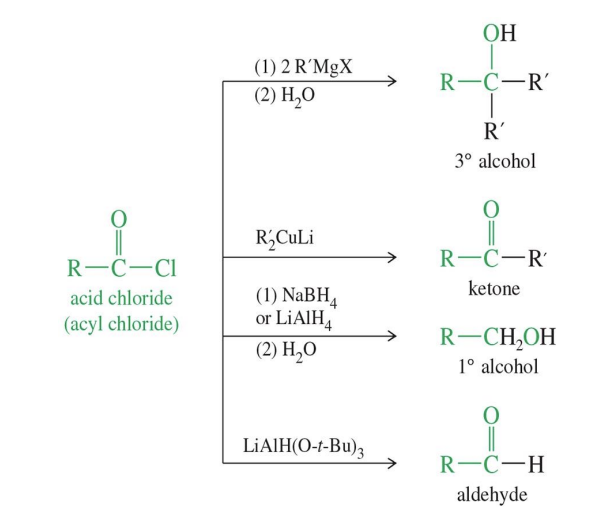
Gringard reagent → to alcohol
Starting
Ester
Acid chloride
Reagent
R - MgX (comes in 2x)
H3O +
Mechanism
the grignard attacks the starting product giving a R group
a tetrahedral intermediate is formed where the double bond of O is single bonded
The OR group leaves forming a Ketone
Another R - mgX comes in and attack the ketone give an R group
FInal product
2 or 3 Alcohol depending on the R group
DIfferent Acid chloride reaction
Friedel crafts acylation
Starting
acid chloride
Benzene ring
Reagent
AlCL3
H2O

FInal product
Ketone
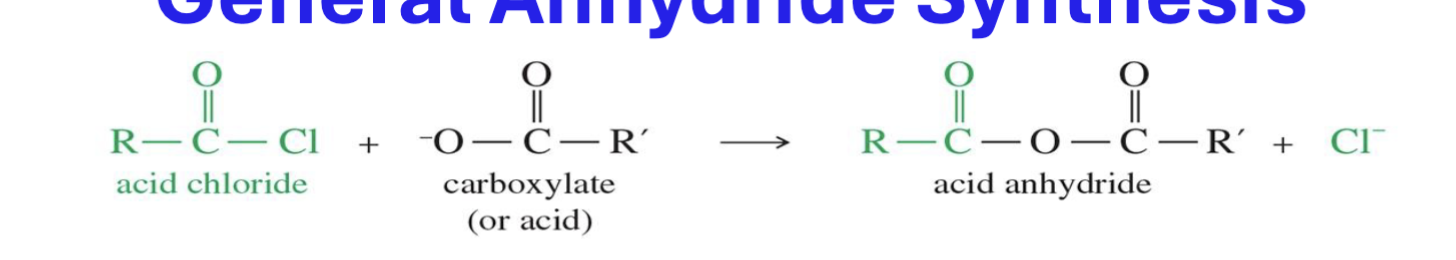
Anhydride Synthesis
Starting
Acid Chloride
Carboxylate
Carboxylic Acid
Reagent :
pyridine
Reaction of Anhydrides
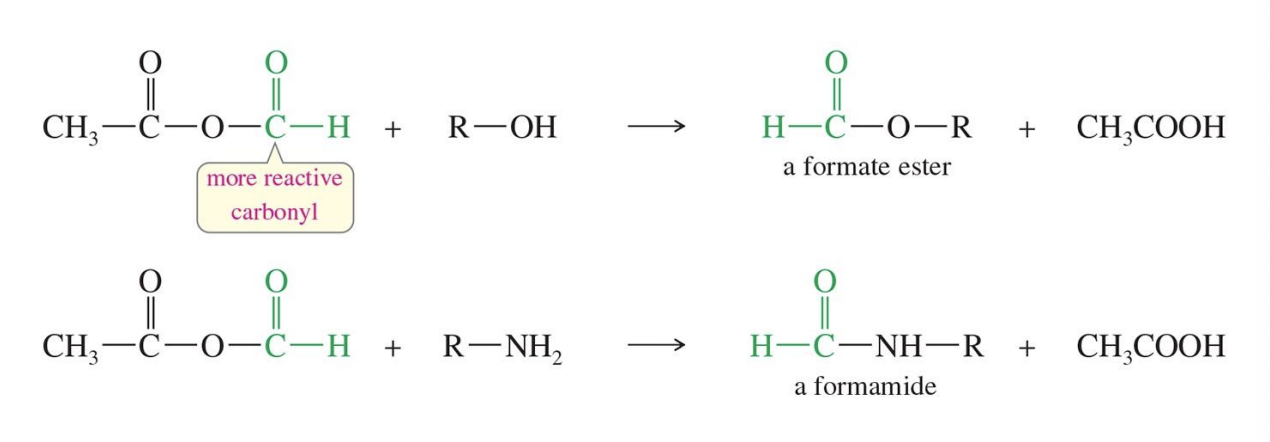
Acetic Formic Anhydride
Starting
Acetic Formic Anhydride CH₃-C(=O)-O-C(=O)-H)
Reagent
R - OH
R - NH2
End
Ester
Amide
Carboxylic Acid
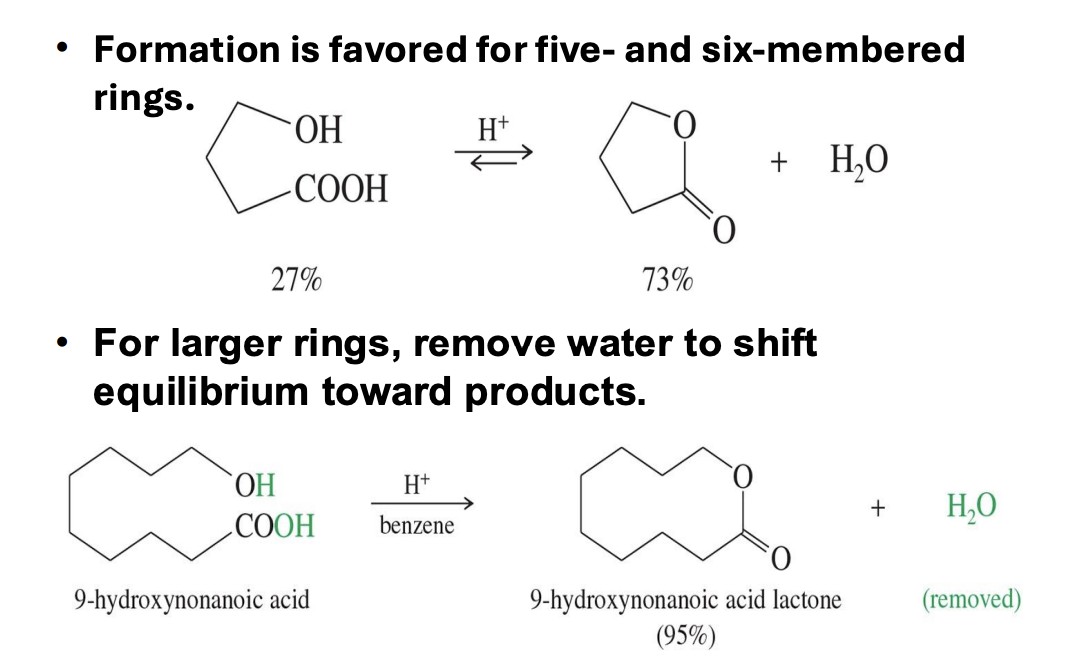
lactone formation
Starting :
hydroxy acid (a molecule with both -OH and -COOH groups)
Reagents:
5-6 membered rings: Form easily with just H⁺
7+ membered rings: Needs H+ And Benzene
End product:
Lactrones : cyclic esters formed from hydroxy acids
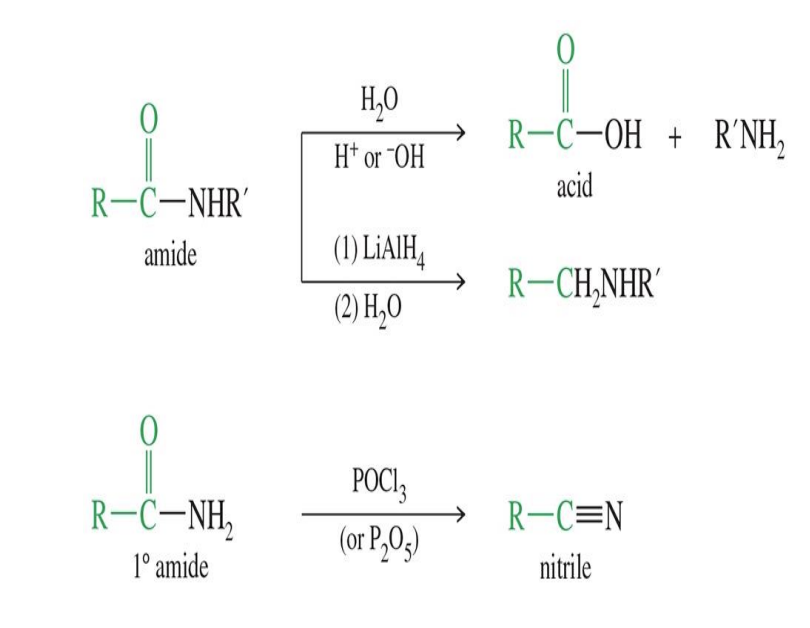
Reactions of Amides
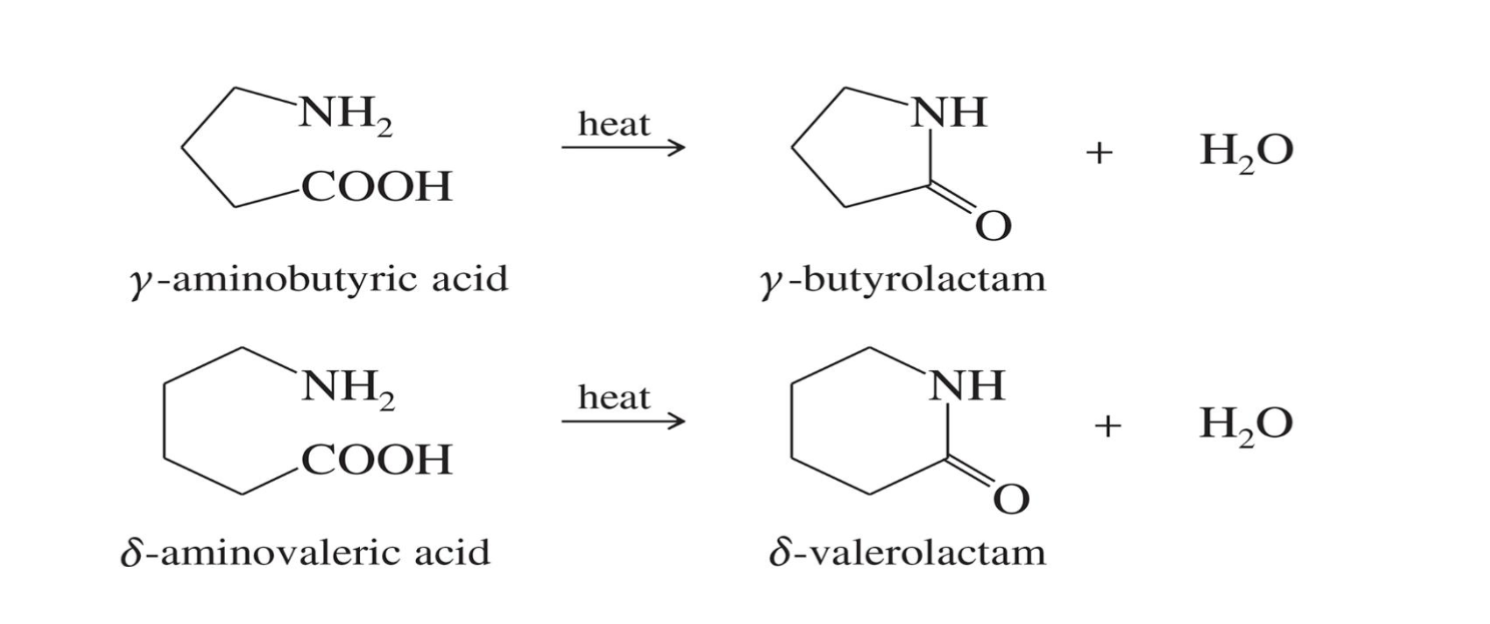
Formation of Lactams
Starting :
An amino acid (a molecule with both -NH₂ and -COOH groups
Reagent
Heat
End product
Lactam → cyclic amide
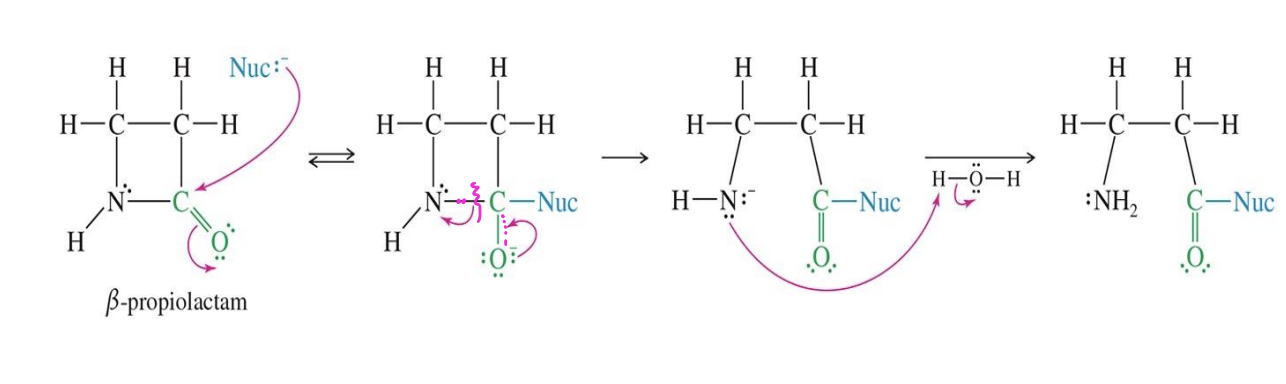
Mechanism of B-Lactam Acylation
Starting material:
β-propiolactam (a 4-membered ring lactam with a C=O)
Reagent:
Nuc: (a nucleophile — could be water, an amine, an alcohol, etc.)
Water
Mechanism
the Nuc attacks the Carbon with the double bonde with Oxygen
The bond breaks between N and C
Water comes in and donates a Hydrogen to the Nitrogen (protonation)
End product
Open-chain amide with a protonated amine group → acylated product
Thioesters formation
Starting
Carboxylic Acid
Reagent
Thiol ( R - SH)
Mechanism
Thiol attacks the carbonyl carbon
Oxygen gets protonated to make water (a good leaving group)
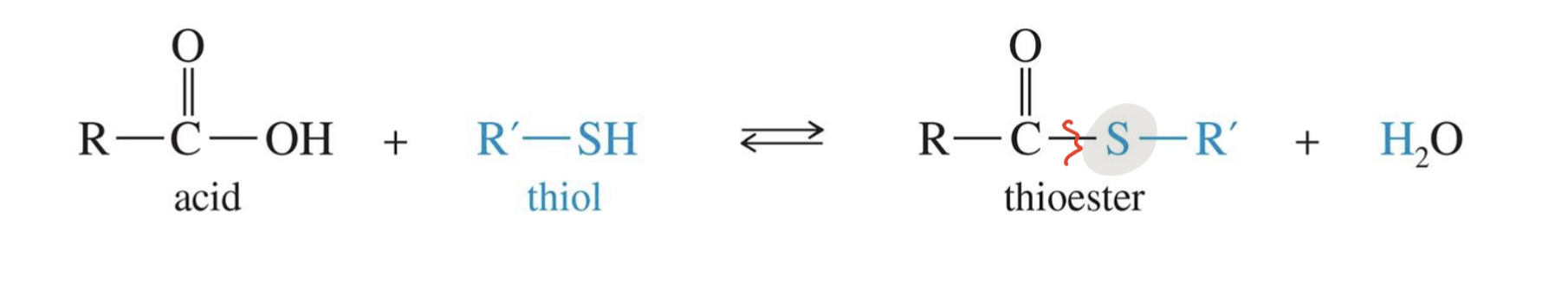
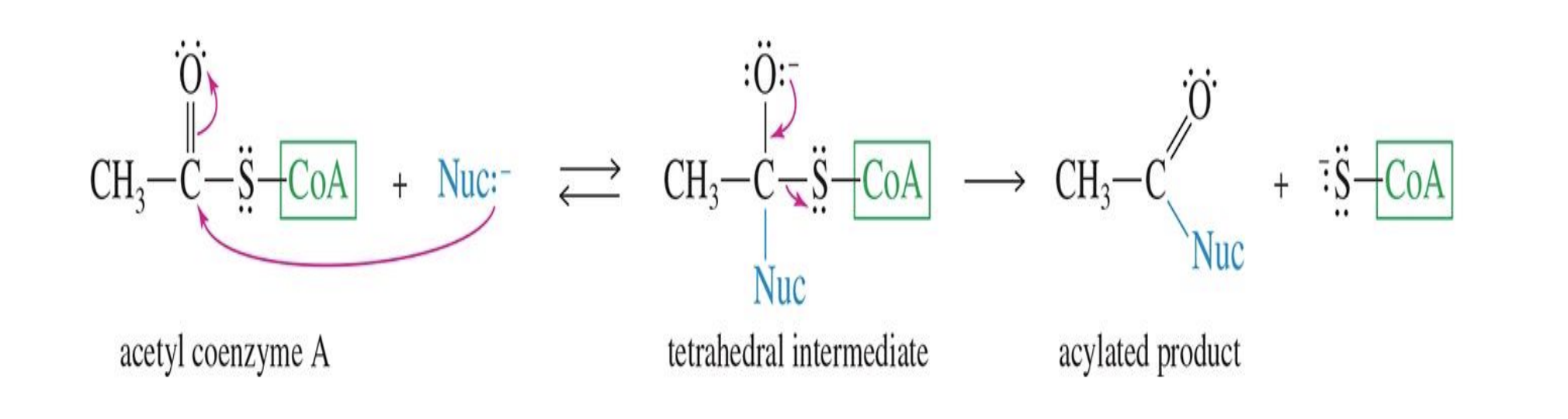
Mechanism of Action of Acetyl CoA:
Starting :
Acetyl CoA (acetyl coenzyme A) CH₃-C(=O)-S-CoA
Reagent:
Nuc: (a nucleophile — could be an -OH, -NH₂, -SH, or another nucleophile)
Mechanism
Nucleophile attacks the carbonyl carbon of acetyl CoA → forms a tetrahedral intermediate
CoA leaves as the leaving group
Acylated product forms — the nucleophile now has an acetyl group attached
End product
acylated product (CH₃-C(=O)-Nuc)
Plus free coenzyme A (CoA-SH)