chapter 4: organic chemical reactions
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
what occurs in the first step of the free radical chain reaction?
initiation: radicals are generated
what are radicals?
species with unpaid electrons that are very reactive
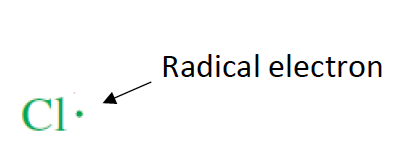
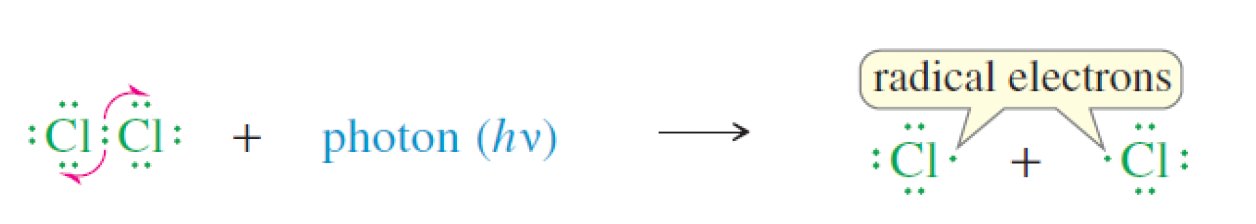
what occurs in the initiation step of the chlorination of methane?
a photon of light is absorbed by Cl2, resulting its split into two Cl free radicals
what occurs in the second step of the free radical chain reaction?
propagation: radical reacts with stable molecule to form a new radical species until the supply is exhausted
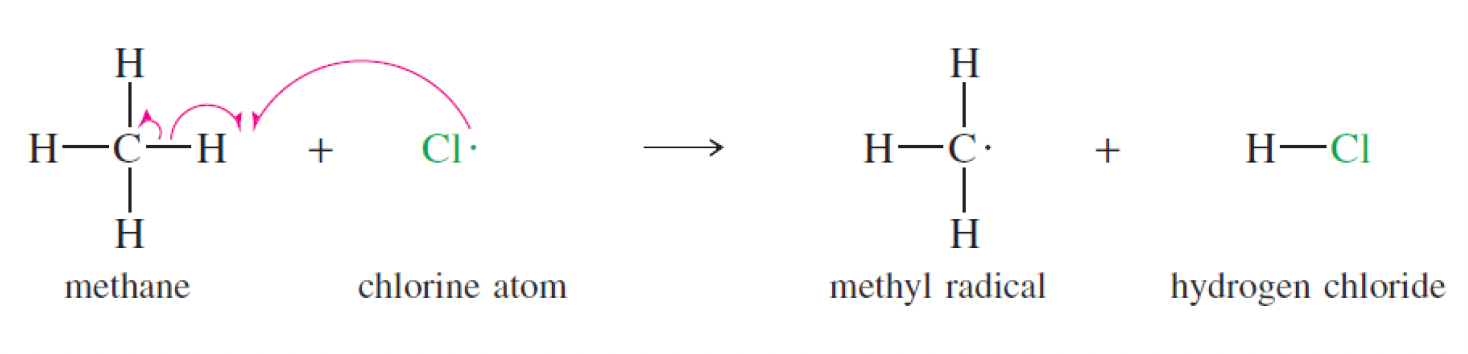
what occurs in the first propogation step of the chlorination of methane?
Cl radical obtains octet by reacting with hydrogen to form HCl, making methane a methyl radical
what occurs in the second propogation step of the chlorination of methane?
methyl radical forms chloromethane while creating new Cl radical
what occurs in the last step of the free radical chain reaction?
radicals are consumed and the propagation of the chain reaction is stopped
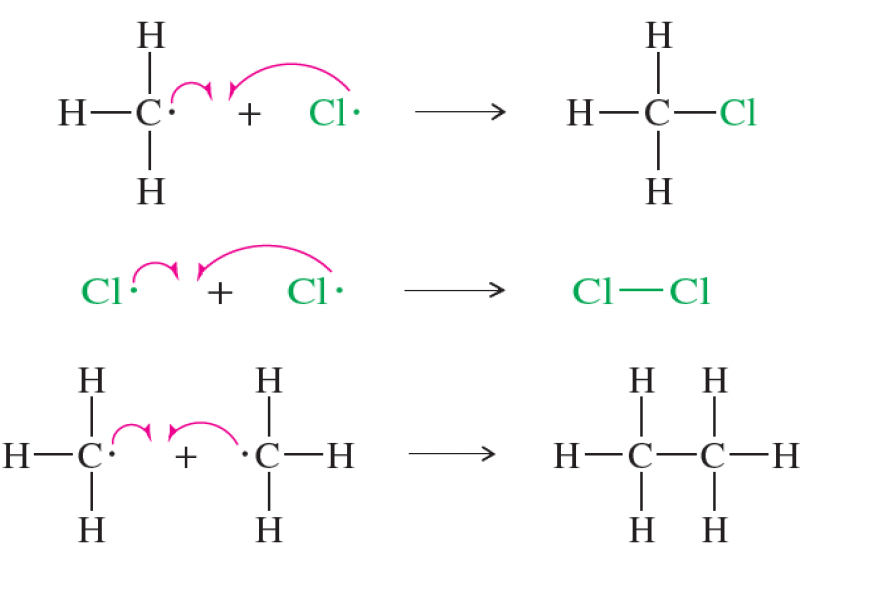
what kind of termination steps can occur in the chlorination of methane?
the combination of any two free radicals
reaction of free radicals with the walls of the vessel
reaction of the free radicals with the contaminants
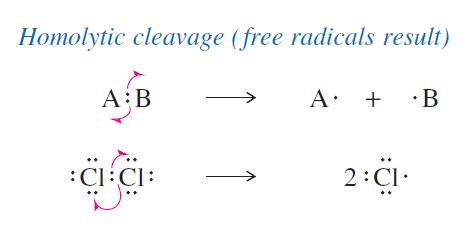
explain what a homoloytic cleavage is
forms free radicals; when bonds break each atom gets one electron
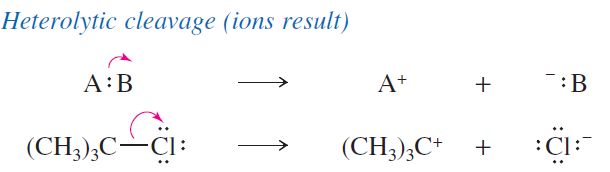
explain what a heterolytic cleavage is
forms ions; when bond break the most electronegative atom gets both electrons