Exothermic & Endothermic Reactions
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
What is an exothermic reaction?
A reaction that transfers energy to the surroundings
What is an endothermic reaction?
A reaction that takes in energy from the surroundings
What is a reaction (or enthalpy) profile diagram?
A diagram that shows the energy change during a chemical reaction
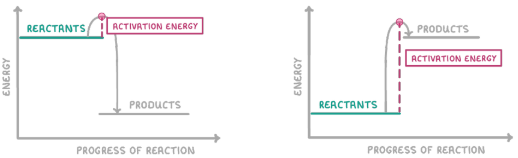
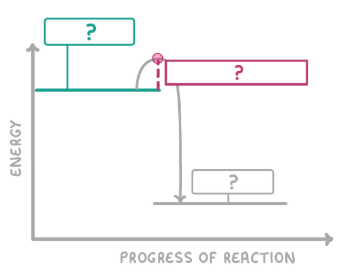
What is the green box?
Reactants

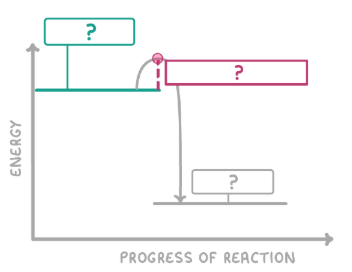
What is the red box?
Activation energy

What is the grey box?
Products

Give 3 examples of exothermic reactions
Combustion
Neutralisation
Oxidation
Give 2 examples of endothermic reactions
Citric acid + sodium hydrogen carbonate
Thermal decomposition
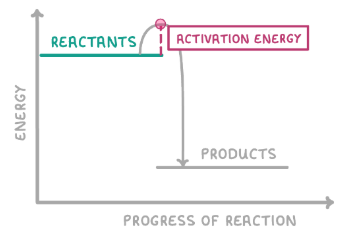
Does the graph show an exothermic or an endothermic reaction? Why?
Exothermic as the energy of products is lower than reactants so energy has been lost to surroundings
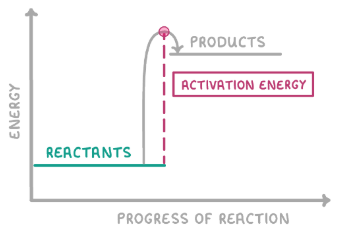
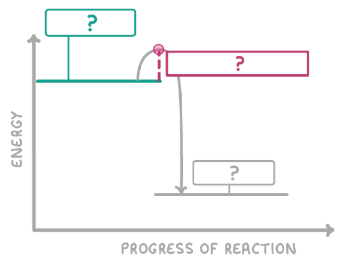
Does the graph show an exothermic or endothermic reaction? Why?
Endothermic as the energy of products is higher than reactants so energy has been gained from surroundings
Do hand warmers and self heating cans use exothermic or endothermic chemical reactions?
Exothermic as they increase the temperature of their surroundings
Do sport injury cold packs use exothermic or endothermic chemical reactions?
Endothermic as they decrease the temperature of their surroundings