Lecture 15: Major CNS NT Synapses and Their Receptors:
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
Neurons in the Human CNS
Contains ~86 ×109 neurons
Glutamate (glutamatergic):↑70%;60 x 109
GABA (GABAergic): ↑30%; 26 x 109
Use GABA/ glycine as a NT
“Neuromodulators”: <0.1%
Dopamine neurons: 400-600 x 103
5-HT (serotonin) neruons: 300 x 103
ACh (Nucleus Basalis Meynert) neurons: 200 x 103
Noradrenaline: 20-50 x 103
Important systems that are targted neuropharamcologically to modulate the nervous system
Synapses per neuron in the brain - Between 1014 and 1015 (100 trillion - 1 quadrillion)
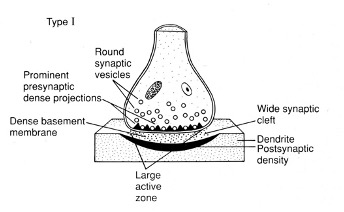
Grey Type 1 Synapse
Asymmetrical structure
Round vesicles
Large active zone – dense in protein and release machinery
Prominent and dense ECM between the pre-synaptic and post-synaptic membrane
The postsynaptic membrane is protein-dense
Excitatory” - associated with L-glutamatergic synapse markers
(antibodies to L-glutamate) – label the synapses exclusively
Why is It Hard to Find Synapses for Neuromodulatory Substances
They are released through volume transmission → released into the extracellular space and influence the surroundings
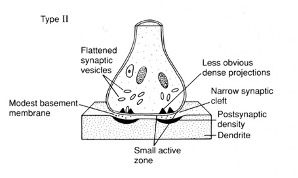
Grey Type 2 Synapse
Symmetrical structure
Flattened vesicles – contain GABA/ glycine
Less prominent active zone
Lower postsynaptic densities
Less prominent extracellular matrix/ base membrane
“Inhibitory” - associated with GABAergic and glycinergic
Dependent on area of the NS
synapse markers (antibodies to GABA)
Excitatory CNS Neurotransmitters
ubiquitous (“everywhere”)
act between the cerebral cortex → spinal cord
Make up 60-70% of all synapses
Inhibitory CNS Neurotransmitters:
γ-Aminobutyric acid (GABA) - cerebral cortex → brain stem
Glycine - brain stem → spinal cord
20-30% of all synapses
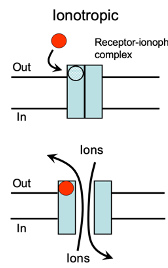
Ionotropic Receptors
Both excitatory and inhibitory neurotransmitters act on these receptors.
They modify the membrane potential by allowing ions to move across the membrane.
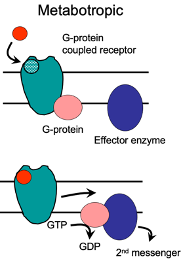
Metabotropic Receptors
Involve activation of G-proteins.
G-protein activation influences various effector targets, such as ion channels.
These ion channels modify cell excitability or influence presynaptic release processes.
Excitatory Neurotransmission
Mediated by glutamate
Glutamate has an excitatory effect when applied to neurons, resulting in the depolarisation of the membrane and AP firing
Applicated to a VC neurons - generation of an inward current, that drives depolarisation
These responses are said to be mediated by the ionotropic receptors
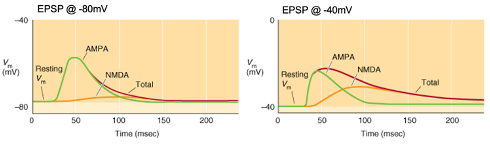
Voltage-dependence of fast excitatory neurotransmission
Activation of a synapse generates a triangular-shaped depolarisation with a rapid rise, peak, and slow decay as the neurotransmitter diffuses away.
Glutamatergic neurotransmission involves two main receptor types (AMPA & NMDA) with voltage dependence affecting the shape of the EPSP.
Graphs: Membrane potential typically at -80mV but can change due to synaptic activity.
Control condition: No antagonists; EPSPs show a rapid rise with a longer decay if the duration is prolonged.
AMPA (green): EPSPs mediated by AMPA receptors in the presence of NMDA antagonists (e.g., D-AP5).
NMDA (orange): EPSPs mediated by NMDA receptors in the presence of AMPA antagonists (e.g., NBQX).
Voltage-dependence: NMDA receptors have a larger role at depolarized membrane potentials, contributing more to EPSPs at higher membrane potentials.
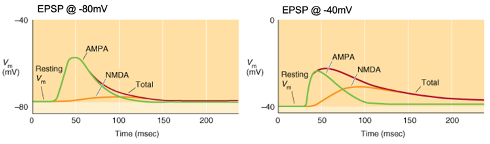
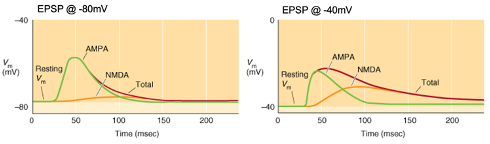
Explain the Graph:
Control condition: No antagonists; EPSPs show a rapid rise with a longer decay if the duration is prolonged.
AMPA (green): EPSPs mediated by AMPA receptors in the presence of NMDA antagonists (e.g., D-AP5).
NMDA (orange): EPSPs mediated by NMDA receptors in the presence of AMPA antagonists (e.g., NBQX).
Voltage-dependence: NMDA receptors have a larger role at depolarised membrane potentials e.g. -40mv, contributing more to EPSPs at higher membrane potentials.
AMPA Receptor Electrophysiology
At 0mV: No response seen (ions at equilibrium potential).
Further depolarisation: Voltage responses reverse and become hyperpolarized.
Under voltage clamp (below 0mV):
Inward currents support EPSPs.
At more depolarized potentials, reversal is seen (no net current during EPSP generation).
At positive depolarized levels, outward current generated → move away from Eion
It is a non-specific cation channel (shown by GHK equation).
At 0mV: Inward and outward ion fluxes balance (net current = 0).
Linear IV plot: No voltage dependence (Ohmic behavior when activated).
linear conformist channel when activated
Voltage Dependence of NMDA-Receptors
I/V Relationship :
Depolarisation to -30mV results in a larger EPSC than at resting membrane potential (-70mV).
Non-linear response — receptors are “deviant” or “non-conformist” and are targets for drugs like ketamine and ethanol.
IV Relationship Distortion:
Above -30mV, NMDA receptor responses resemble AMPA receptors.
Below -30mV: the response becomes non-linear, deviating from Ohm's law (less current, indicating an interference with ion flow at hyperpolarised potentials).
Current through NMDA receptors is linear around 0mV, and the ion flow reverses around this point, following Ohm's law in this range.
Effect of Removing or Decreasing Mg2+ From Bathing Solution of NDMA Receptors on Current Relationship
ohmic relationship seen - linear plo
Mg2+ Block Of NMDA-Receptors
Mg2+ acts as a channel blocker by becoming trapped in the open NMDA receptor channel at hyperpolarised potentials, preventing ion flow.
block occurs at hyperpolarised potentials after ligand binding and receptor activation to block ion flow.
At depolarised potentials, the electrostatic Mg²⁺ block weakens, allowing ions to flow through the channel, resulting in linear (ohmic) behaviour.
Similarity in NMDA and K+ Channels
Evolutionarily similar as both are tetramers.
However, Mg²⁺ blockage occurs in the opposite direction for NMDA receptors compared to K⁺ channels.
Ionotropic Glutamate Receptors (iGluRs)
The receptor subunits are categorised by sequence homology.
The closer they are on the homology tree, the more similar their amino acid sequences
These functional receptors are
tetramers (4 subunits) with 4 agonist-binding sites.
Homomeric or heteromeric: Difficult to identify subunits in situ.
Non-selective cation channels (K+, Na+, Ca2+) with Eion ~ 0mV.
Types of iGluRs: AMPA Receptors
GluA1-4: 4 different subunits
Receptor can be homomeric or heteromeric - difficult to identify subunits in situ
Named after its agonist agonist
Types of iGluRs: NMDA Receptors
GluN1-2 → Complex subunits with dual agonism.
GluN1: Glycine binding site.
GluN2: Glutamate binding site.
Requires both glycine and glutamate for activation.
Heteromeric
Types of iGluRs: Kinate Receptors
GluK1-5 subunits
Receptors may be homomeric or heteromeric - difficult to identify in situ
Named after agonists
limited distribution.
Postsynaptic Electrophysiology: Group I mGluRs
Glutamate acts on inotropic and metabotropic receptors (mGluRs) at the postsynaptic terminal.
Agonist: Dihydroxyphenylglycine (DHPG)
Selective for mGluRs; can cause membrane depolarisation and AP firing.
TTX (Tetrodotoxin) is used to block Na+ channels to assess excitation and block AP generation.
DHPG modulates ionotropic receptors (e.g., NMDA receptors) by increasing depolarisation and enhancing excitability.
Three classes of metabotropic glutamate receptors are involved in post-synaptic effects and regulation of excitatory neurotransmission.
Pre-synaptic Electrophysiology Group II mGLuRs
The receeptor regulates the function and causes a depression of release
mGluR agonist: DCG-IV
Depresses synaptic transmission through a pre-synaptic action
Reduces the amplitude of responses
Affects the mossy fibre pathway (dentate gyrus → area CA3)
Pre-synaptic Electrophysiology Group III mGLuRs
The receptor regulates the function and causes a depression of release
mGluR agonist: L-aminophosphonobutyrate (L-AP4)
Depresses synaptic transmission through a pre-synaptic action
Reduces the amplitude of responses
Metabotropic Glutamate Receptors (mGluRs)
The receptor subunits are categorised by sequence homology.
The closer they are on the homology tree, the more similar their amino acid sequences
There are 8 subtypes of receptor
3 Principal receptor groups include
Group 1: Postsynaptic
Groups 2 and 3: Presynaptic
These receptors function as homodimers and consist of:
Ligand binding domain: Recognizes glutamate.
7 TM domains,
C-terminal intracellular loop.
Dimer formation creates a functional receptor that activates G-proteins.
Effector Targets of Group I mGlu-Rs (Post Synaptic)
These mGluRs directly modify channel function via activation of PKC via the αq11 subunit of a G-protein.
This activation increases PLC (phospholipase C), generating DAG and IP3, which further increase PKC.
PKC phosphorylation of Tandem 2-pore domain K+ channels (K2P) results in their closure → depolarisation.
NMDA-R current is enhanced by PKC phosphorylation, leading to increased NMDA receptor-mediated currents.
Effect: Enhanced excitability and increased responsiveness to NMDA signaling.
Effector Targets of Group II & III mGlu-Rs (Pre-synaptic)
Target Ca2+ channels, decreasing their function
Target P/Q- and N-type (CaV2.1-2) Ca2+ channels, reducing neurotransmitter release.
Mechanisms:
Direct: G-protein βγ dimer interacts with Ca2+ channels to inhibit function.
Indirect: αi subunit → ↓ cAMP → ↓ PKA → enhances phosphatase activity → dephosphorylation of Ca2+ channels.
Result:
Decreased likelihood of neurotransmitter release and synaptic responsiveness → decrease in excitabliity
Modified excitability due to shift in balance towards dephosphorylation of Ca2+ channels.
Key events at a glutamatergic synapse
Early fast EPSP: Mediated by ionotropic receptors (AMPA or AMPA+NMDA), dependent on voltage.
Delayed/late EPSP: Initiated by the closure of K+ channels due to activation of postsynaptic mGluRs.
K+ channel closure: Mediated by the B-y dimer; phosphorylation plays a role.
NMDA upregulation: Phosphorylation increases NMDA channel activity, leading to augmented responses.
Glutamate releasectivates ionotropic receptors on the postsynaptic density, opposite the release site.
mGluRs outside the postsynaptic density: Can modify excitability.
Autoreceptors on presynaptic terminal: Modulate NT release; feedback loop when presynaptic terminal is repeatedly activated.
Inhibitory Neurotransmission
GABA: Primary _______ neurotransmitter in the forebrain.
Glycine: Major ______ neurotransmitter in the spinal cord and brainstem.
Both act at ionotropic receptors to hyperpolarise the membrane potential, preventing neuron firing
GABA acts at a subset of metabotropic receptors
These neurotransmitters hyperpolarise the membrane potential, inhibiting an active neuron from firing.
Hyperpolarisation occurs when activation of receptors causes an outward current, moving the MP toward a more negative, hyperpolarised state
Ionotropic GABA/Glycine Receptor Electrophysiology
These channels are anion-selective channels - Cl-; Eion ~ -70mV
Above -70mV: hyperpolarisation that increases as the membrane potential moves further from Eion
Outward current → upward deflection → Inhibitory Post-Synaptic Potential (IPSP)
Below -70mV:
Activation of receptors can lead to depolarisation (moves MP toward -70mV)
Inward current → downward deflection
Potential for excitatory effect (debated)
Effect is primarily inhibitory → break of activity, prevents AP firing
Outward current → chloride influx (Cl-) at hyperpolarised states
Inward current → chloride efflux
IV plot characteristics:
Linear, ohmic relationship
Constant conductance (channels always open
GABA-A receptors
Ionotropic receptor: Part of the same gene superfamily as glycine receptors.
Structure: Pentameric (five subunits), typically composed of 2 α-, 2 β-, and 1 γ-subunit.
Pharmacology: Wide variety due to different subunits (e.g., α- and β- subunit interface where GABA binds).
Different sensitivities do different neuromodualtors
Function: Opens Cl⁻ channels, producing an inhibitory effect.
Drugs: Target the α-β interface to modify GABA activation.
Glycine Receptors
Ionotropic receptor: Related to GABA-A receptors, part of the same gene superfamily.
Structure: Pentameric, composed of alternating A- and B-subunits (B-A-B-A-B stoichiometry ).
Function: Inhibitory, opens Cl⁻ channels to exert effects.
Shares functional characteristics with GABA-A receptors.
GABA-C Receptors
Ionotropic receptor: Structurally distinct from GABA-A receptors.
Subunits: Composed of Rho subunits.
Function: Produces inhibitory effects in various CNS regions by opening Cl⁻ channels.
Distinct subunit composition compared to other ionotropic receptors
GABA-B Post Synaptic Electrophysiology
Agonist: Baclofen
Results in hyperpolarisation with a longer time course
Due to the activation of metabotropic receptors
Forms part of the IPSP (Inhibitory Postsynaptic Potential)
Effect of GABA-B antagonist:
Late phase of IPSP disappears
EPSP shape speeds up
This shows that GABA-B receptor-mediated hyperpolarization contributes to the inhibitory synaptic response.
2 Phases of IPSPs
Fast phase
Slow phase
GABA-B Pre-Synaptic Electrophysiology
Inhibition of IPSP
Reversible depression observed with baclofen application and its subsequent removal
Regulates of glutamatergic function at glutamatergic terminals
Baclofen (agonist) reduces the size of glutamate EPSP
Receptors regulate both inhibitory and excitatory synaptic transmission across a wide range.
Metabotropic GABA-B Receptors
Composed of 2 different subunits that form a complex arrangement → can’t function independently
GABAB1-R subunits: agonist binding site for GABA
GABAB2-R subunits: signals to G-proteins
Receptors form a dimer of heterodimers, requiring both subunits to be functional
2 agonists binding sites must associate with the effector subunits to activate the G-protein
Affect target similarly to metabotropic glutamate receptors:
Target K+ ion channels (post-synaptic effects, membrane potential regulation).
Target Ca2+ channels (pre-synaptic effects, modulation of neurotransmitter release).
GABA-B Receptors - Post Synaptic Effector Targets
The receptors are directly liked to the post-synaptic Kir channels via the B-y dimer, following the activation of the G-protein and receptor
Activation of G-protein coupled to inward rectifier K+ channels (GIRKS) → Kir 3.1-4 and upregulates its functionality – greater movement of K+ ions and a greater effect on MP
increase channel function
Postsynaptic → hyperpolarization
Reduced excitabilit
GABA-B Receptors: Pre Synaptic Effector Targets
decrease pre-synaptic calcium channel function.
Mechanisms:
Direct: βγ dimer of G-protein interacts with P/Q- and N-type Ca²⁺ channels (CaV2.1 & CaV2.2) → ↓ Ca²⁺ entry → ↓ neurotransmitter release.
Indirect: αi subunit of G-protein ↓ cAMP → ↓ PKA → dephosphorylation of Ca²⁺ channels → ↓ channel functionality.
Balance between protein kinases and phosphatases shifts in favour of dephosphorylation of Ca2+ channels and reduces their functionality
Result: Decreased channel activity reduces liklihood of neurotransmitter release, leading to inhibitory effects on neurotransmission and modifying excitability.
Key Mechanisms in Inhibitory Neurotransmission
Early IPSP: Mediated by GABA-A receptors.
Late IPSP: Mediated by GABA-B receptors, which are coupled to inward rectifier channels causing inhibition.
Pre-synaptic autoreceptors: Located on inhibitory presynaptic terminals, reduce neurotransmitter release by inhibiting the release process.
GABA can escape the synapse and inhibit neighbouring excitatory synapses, reducing glutamatergic neurotransmission.
Interplay between excitatory and inhibitory influences in major NT synapses in the CNS
EPSP: Depolarises the membrane. If large enough, it reaches the threshold and triggers an action potential (AP).
IPSP: Hyperpolarises the membrane, pushing the membrane potential closer to -70mV, inhibiting action potential generation.
Combined Activation: Excitatory and inhibitory signals together regulate the neuron’s approach to the firing threshold, balancing excitation and inhibition.
inhibitory inputs can regulate the excitatory inputs and modify the neurons approach to threshold
Two ways interneurons modify excitatory function:
Direct influence: Excitatory input on a principal neuron can trigger an EPSP and activate an inhibitory interneuron, to produce an IPSP.
Feedback influence: Excitatory input. can activate the principal neuron, which can influence inhibitory interneurons to feedback and modify subsequent excitatory responses and neurons.
Feedforward and feedback inhibition: Present to regulate neuronal network excitability.
Prinicpal Cells in Corical Circuits
Principle cells are wired to have reciprocal excitatory connections as well as feedforward and feedback inhibitory connections
Intact inhibition: excitability of the system is low, with activation of excitatory inputs generating a modest response
Removal of inhibition: excitation propagates in the cortical circuit, between principal cells, allowing for a long-lasting excitatory response which is sustained over a period of time (in response to a single input)
Importance of Inhibition in Cortical Excitabilitiy
It controls cortical excitability.
Without it, overexcitation can occur, leading to epilepsy.
In particular cortical areas and the nervous system, inhibition is crucial for preventing sustained excitatory circuits that can trigger seizures.
Competitive antagonists of GABAA receptors (e.g., Bicuculline) and Glycine receptors (e.g., Strychnine) block inhibition, leading to convulsions and seizures due to overexcitation.