APCHEM Unit 2 Test
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
Rank the bonds formed between O–C, Cl–Ca, N–S, and O–Si in order of increasing ionic character.
N–S < O–C < O–Si < Cl–Ca
N–S < O–C < Cl–Ca < O–Si
Cl–Ca < O–Si < O–C < N–S
O–C < N–S < O–Si < Cl–Ca
N–S < O–C < O–Si < Cl–Ca
On the basis of the information in the table, which of the following arranges the binary compounds in order of increasing bond polarity?
Element | Electronegativity |
H | 2.1 |
C | 2.5 |
S | 2.5 |
F | 4.0 |
Cl | 3.0 |
Si | 1.8 |
CH4 < SF4 < SiCl4
SiCl4 < SF4 < CH4
SF4 < CH4 < SiCl4
CH4 < SiCl4 < SF4
CH4 < SiCl4 < SF4
Why does MgCl2 (2326 kJ/mol) have a stronger lattice energy than LiCl (834 kJ/mol)?
There are twice as many chlorine ions in MgCl2 compared to LiCl and it requires more energy to make twice as many bonds.
The Mg ion has a 2+ charge which is twice the charge of the lithium ion, causing the attraction with the chlorine ion to be stronger.
The Li ion has a smaller ionic radius that the Mg ion so it has a weaker attraction to the chlorine ions
The Mg ion has a larger ionic radius than the Li ion so it has a stronger attraction to the chlorine ions
The Mg ion has a 2+ charge which is twice the charge of the lithium ion, causing the attraction with the chlorine ion to be stronger.
What is the main factor contributing to the difference in lattice energies in the lithium halogen compounds on the table?
Bond | Lattice Energy (KJ/mol) |
LiF | 1030 |
LiCl | 834 |
LiI | 730 |
The bond order
The electronegativity values
The size of the halogen ion
The charge of the ions
The size of the halogen ion
The melting point of MgO is higher than that of NaF. Explanations for this observation include which of the following?
I. Mg2+ is more positively charged than Na+.
II. O2- is more negatively charged than F-.
III. The O2- ion is smaller than the F- ion.
I, II, and III
I and III only
I and II only
II only
II and III only
I and II only
The potential energy of a system of two atoms as a function of their internuclear distance is shown in the diagram. Which of the following is true regarding the forces between the atoms when their internuclear distance is x?
It cannot be determined whether the forces between atoms are balanced, attractive, or repulsive, because the diagram shows only the potential energy.
There is a net attractive force pulling the atoms together, so the atoms will move closer together.
There is a net repulsive force pushing the atoms apart, so the atoms will move further apart.
The attractive and repulsive forces are balanced, so the atoms will maintain an average internuclear distance x.
The attractive and repulsive forces are balanced, so the atoms will maintain an average internuclear distance x.
Using the table of lattice energies, predict which ionic compound will have the highest melting point?
Ionic Compound | Lattice Energy (kJ/mol) |
NaBr | 732 |
NaF | 910 |
NaI | 632 |
NaCl | 765 |
NaF
NaI
NaCl
NaBr
NaF
All of the following are properties of solid ionic compounds, EXCEPT:
Conducts electricity
Dissolves in water
Forms a crystal lattice
Brittle structure
Conducts electricity
Solids have many different properties. _________ solids are known for their ability to be flattened into a sheet, stretched into a wire, and to conduct electricity well.
network
metallic
molecular
ionic
metallic
The characteristic of covalent bonds present in the metallic structure is that they are:
delocalized
temporary
localized
permanent
delocalized
What is the hybridization of Br in BrF3?
sp3d2
sp3
sp2
sp3d
sp3d
Which of the following molecules has the shortest bond length?
Br2
I2
N2
Cl2
O2
N2
Which species has a trigonal-pyramidal molecular geometry?
SiH4
BH3
CH4
H2O
NH3
NH3
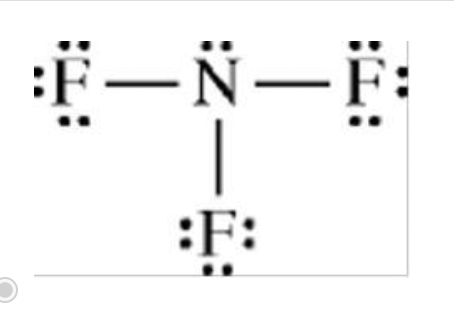
Which of the following Lewis electron-dot diagrams represents the molecule that is the most polar?
incorrect
Which gaseous molecule is a polar molecule
N2
F2
SO2
BeCl2
O2
SO2
Which gaseous molecule is best represented by two or more resonance forms
N2
SO2
F2
BeCl2
O2
SO2
The geometry of the SO3 molecule is best described as
trigonal planar
tetrahedral
trigonal pyramidal
square pyramidal
bent
trigonal planar
Which of the following molecules contains exactly three sigma (σ) bonds and two pi (π) bonds?
SO3
CO2
N2
C2H2
HCN
C2H2
The structural formula of the glycinium cation is shown in the figure. Arrows indicate the pKa values for the labile protons in the molecule. Which statement is true about the geometry of the glycinium cation?
The N – C – C bond angle is 180°.
The leftmost C atom and all the atoms directly bonded to it lie in the same plane.
Both C atoms and both O atoms lie in the same plane.
The geometry around the N atom is planar.
Both C atoms and both O atoms lie in the same plane.
Which of the following has a zero dipole moment?
SO2
HCN
PF5
NH3
NO2
PF5
Which molecule has trigonal pyramidal geometry?
CO2
PH3
H2O
CH4
C2H4
PH3
Which element follows the duet rule?
He
C
Ne
O
He
Select the answer that best fits the statement: Is isomeric with CH3CH2CHO.
CH3CH2CH2CH3
CH3COCH3
CH3CH2CH2NH2
CH3CH2CH2OH
CH3COOH
CH3COCH3
What is the formal charge on each atom in the N2O Lewis structure below, read from left to right?
0, +1, -1
0, 0, 0
0, +1, 0
+1, 0, -1
0, +1, -1
Based on the resonance structures shown, what are the bond orders of the two carbon-oxygen bonds?
1 and 2
2 and 2
1.5 and 1.5
1 and 1.5
1.5 and 1.5
Why do all of the H–C–H bonds in methane have the same bond angle?
Carbon has 4 valence electrons so the hydrogen atoms can bond equally
All of the hydrogen atoms have the same 1s orbital so they all bond the same
All the bonding atoms are identical so they take up the same amount of space
Carbon's valence shell s and p orbitals hybridized to make four identical sp3 orbitals
Carbon's valence shell s and p orbitals hybridized to make four identical sp3 orbitals
What is the molecular geometry of phosphine, PH3?
Tetrahedral
Trigonal Pyramidal
Linear
Bent
Trigonal Pyramidal
Pi (π) bonding occurs in each of the following species EXCEPT
CO2
CN
C2H4
C6H6
CH4
CH4
Of the following molecules, which has the largest dipole moment?
CO
CO2
F2
HF
O2
HF
Which of the following diatomic species has the largest bond-dissociation energy
Li2
F2
B2
O2
N2
N2
Which of the following diatomic species contains 1 sigma (σ) and 2 pi (π) bonds
F2
B2
N2
O2
Li2
N2
Which of the following species is NOT planar?
NO3-
CO32-
ClF3
PCl3
BF3
PCl3
Lewis electron-dot diagrams for CO2 and SO2 are given. The molecular geometry and polarity of the two substances are
different because the lone pair of electrons on the S atom make it the negative end of a dipole
the same because C and S have similar electronegativity values
different because S has a greater number of electron domains (regions of electron density) surrounding it than C has
the same because the molecular formulas are similar
different because S has a greater number of electron domains (regions of electron density) surrounding it than C has
Which of the following is a nonpolar molecule that contains polar bonds?
NH3
CHF3
CO2
F2
HCl
CO2
A Lewis diagram for the molecule C2H4 is shown in the figure. In the actual C2H4 molecule, the H-C-H bond angles are closest to
120°
180°
109.5°
90°
120°
Which of the following molecules is nonpolar but has polar covalent bonds?
H2O
CCl4
N2
CH2Cl2
H2O2
CCl4
Which of the following arranges the molecules N2, O2, and F2 in order of their bond enthalpies, from least to greatest?
O2 < N2 < F2
N2 < O2 < F2
N2 < F2 < O2
F2 < O2 < N2
F2 < O2 < N2
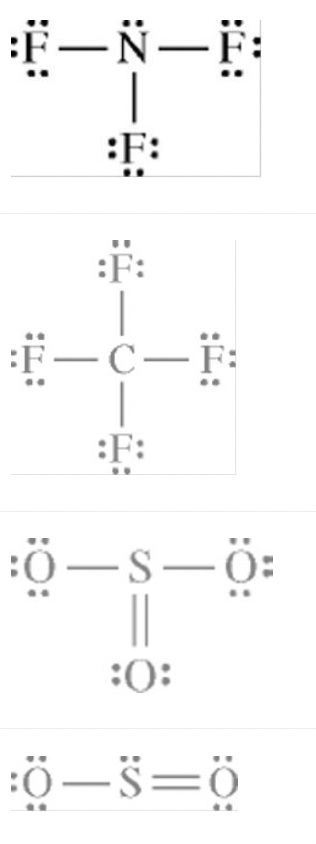
Which of the Lewis electron-dot diagrams represents the molecule that contains the smallest bond angle?
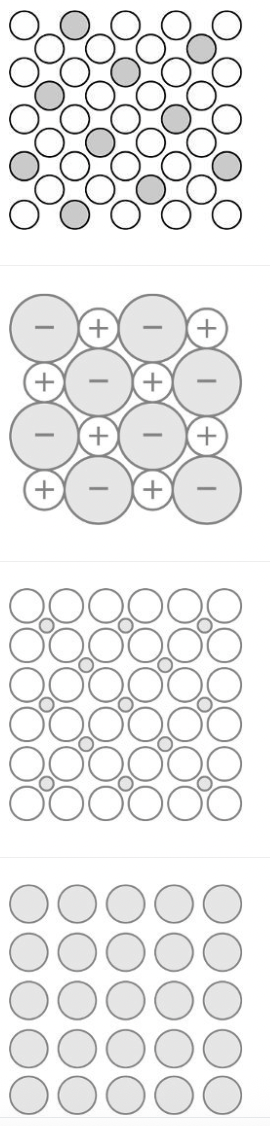
Copper atoms and zinc atoms have the same atomic radius, 135 picometers. Based on this information, which of the following diagrams best represents an alloy containing only copper and zinc atoms?
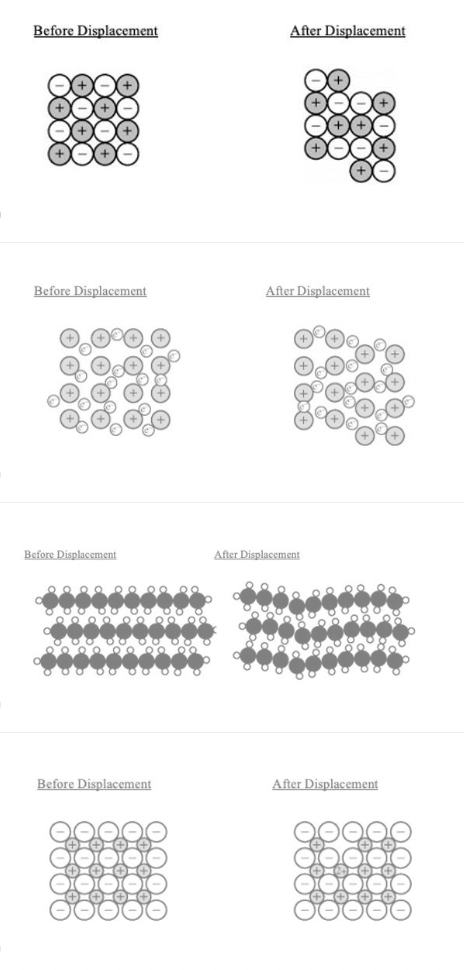
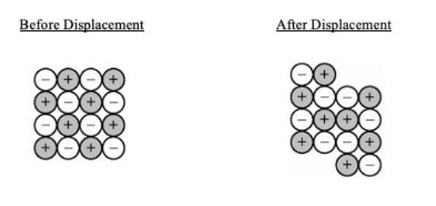
Which of the following diagrams best illustrates how a displacement in an ionic crystal results in cleavage and brittleness?