Equilibrium Constants
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
Collision Theory of Reactions: A chemical reaction occurs when.
Collisions between molecules have sufficient energy to break the bonds in the reactant.
molecules collide with the proper orientation.
Activation Energy
The minimum amount of energy needed for a reaction to take place.
How much energy will the products of an EXOTHERMIC REACTION have?
Products have less energy then it started with.
How much energy will the products of an ENDOTHERMIC REACTION have.
Products have more energy then it started with.
How does temperature effect reaction rate?
The higher the temperature the higher the rate of reaction.
How does concentration effect reaction rate?
The higher concentration, the higher the rate of reaction.
How does a catlyst effect reaction rate.
Speeds up the rate of the reaction and lowers the activation energy (or the amount of energy needed for a reaction to take place.) A catalyst is not used up during the reaction.
How does the nature of the reactant effect reaction rate?
The more surface area the higher the reaction rate.
What is a system at EQUILIBRIUM.
The rate of the forward reactiong is equal to the rate of the reverse reaction.
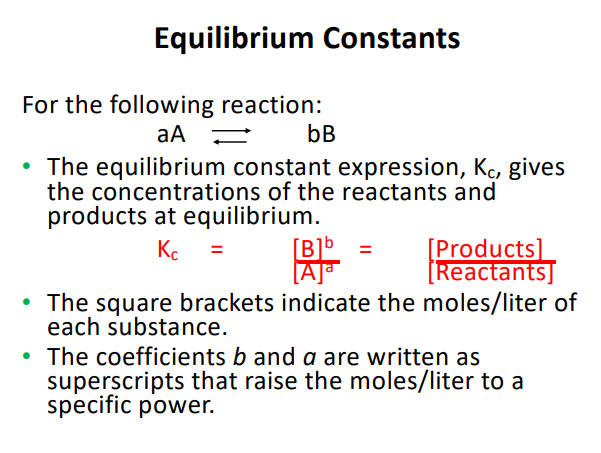
Equilibrium Constant Expression
Kc= Product/Reactant
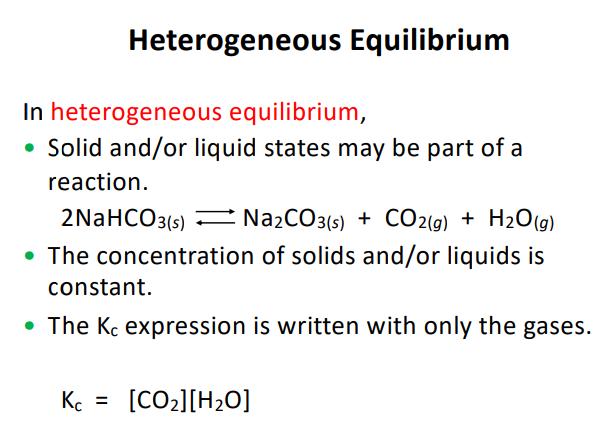
What is the concetration of liquids or solids in a heterogeneous mixture.
They are constant so you can make them 1 in you equations
Equilibrium with a large Kc (more than 1)favors the reactant or the product.
Favors the PRODUCT.
Equilibrium with a small Kc value (less than 1) favors the product or the reactant.
Favors the reactant