Cell Communication and Signaling Pathways
1/40
Earn XP
Description and Tags
These flashcards cover key concepts and terminology from the lecture on cell signaling and communication, focusing on pathways involved in immune response and cancer.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
Modular interaction domains
Different functional sections of proteins that allow for specific binding and signaling.
Phosphotyrosine binding (PTB) domain
A modular domain that attaches to phosphorylated tyrosine residues on activated receptors.
Src homology 2 (SH2) domain
A domain that binds specifically to phosphotyrosine, allowing recognition of specific proteins.
how do you know it is specific?
SH2 binds to amino acids specfic to the their phosphotyrosine on top of
Src homology 3 (SH3) domain
A domain that binds to proline-rich regions of other proteins to assemble signaling complexes.
JAK-STAT signaling pathway
A signaling pathway where cytokine receptors bind JAK, triggering a cascade that activates STAT proteins to regulate gene expression.
This pathway is crucial for the immune response and cell proliferation, involving the activation of Janus kinases (JAKs) by cytokine receptors, which then phosphorylate Signal Transducer and Activator of Transcription (STAT) proteins.
Cytokine
Small proteins that are important in cell signaling, particularly in immune responses.
Receptor tyrosine kinases (RTK)
Proteins that, when bound by a ligand, dimerize and phosphorylate tyrosine residues in their own intracellular domains.
Epidermal Growth Factor (EGF)
A growth factor that stimulates cell growth, division, and differentiation through signaling via its receptor (EGFR).
Ras protein
A small GTPase involved in transmitting signals within cells, activated by EGF signaling.
Mitogen-activated protein kinase (MAPK)
A kinase that is part of a signaling cascade and is activated by Ras to regulate cell growth and division.
Herceptin
An antibody that blocks HER2 signaling by binding to the extracellular domain of HER2.
Lapatinib
A kinase inhibitor that binds to the HER2 cytoplasmic domain and blocks ATP binding, preventing kinase activity.
Autoimmune disease
A condition in which the immune system mistakenly attacks the body's own tissues.
Rheumatoid arthritis (RA)
A chronic inflammatory disorder that primarily affects joints, resulting from an unregulated immune response.
apoptosis
A form of programmed cell death that occurs in multicellular organisms, helping to maintain homeostasis and eliminate damaged cells.
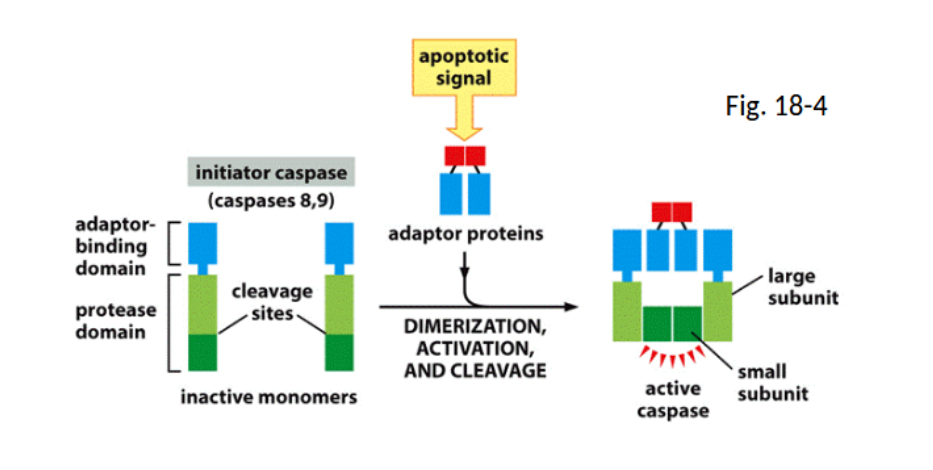
capsases/zymogens
Inactive precursors that, when activated, play a key role in the apoptosis process by executing cell death.
caspase initatior pathway
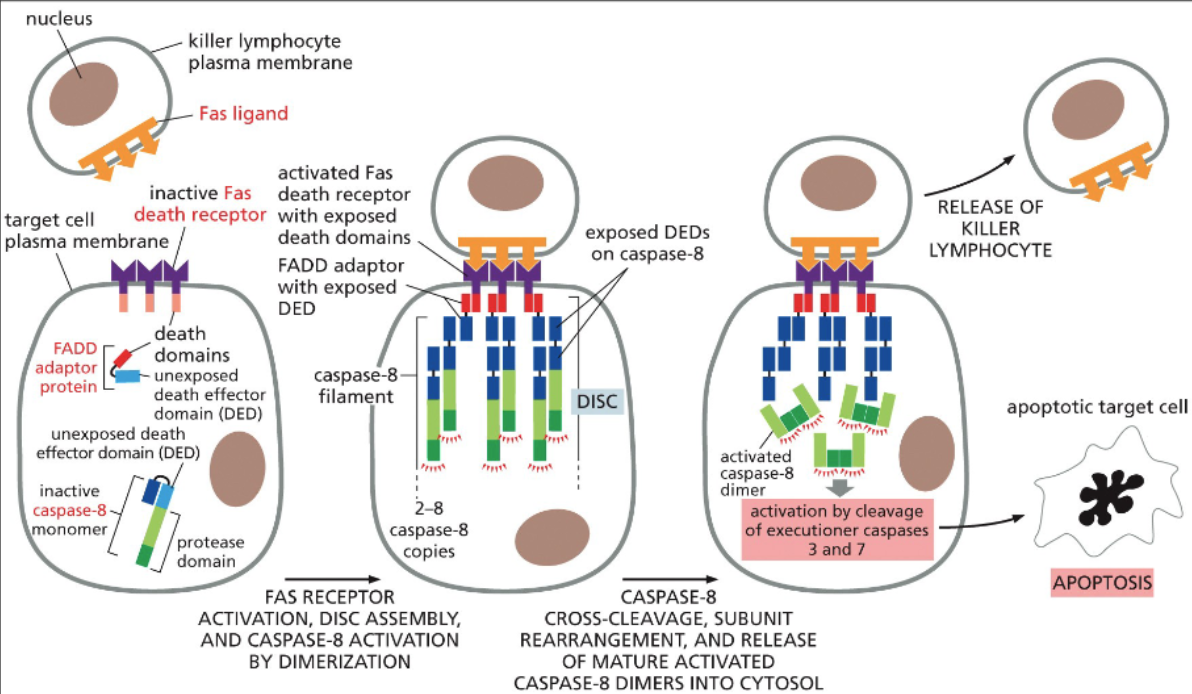
extrinsic aptosis pathway
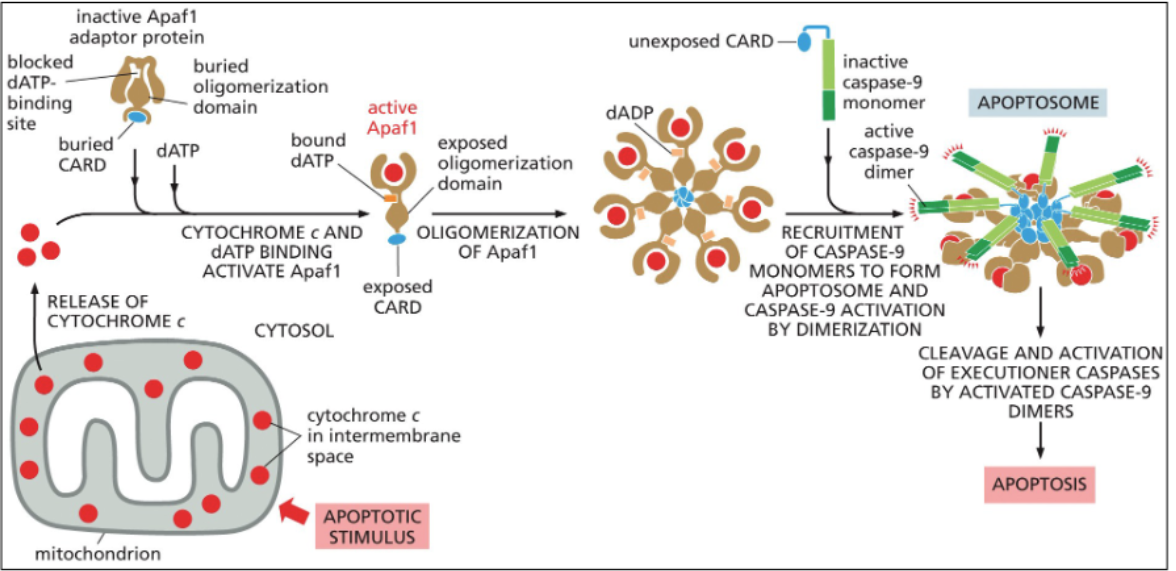
intrinsic apoptosis pathway
what are the key differences between the extrinsic and intrinsic apoptotic pathway??
The extrinsic apoptosis pathway is triggered by external signals binding to death receptors on the cell surface, while the intrinsic pathway is activated by internal signals, such as DNA damage, leading to mitochondrial release of cytochrome c and activation of caspases.
How does the c-FLIP protein shut down the extrinsic pathway?
c-FLIP protein inhibits the activation of caspases by binding to death receptors, thus preventing the apoptotic signal from being transduced through the extrinsic pathway.
Role of Pro-apoptotic Bax & Bak proteins
Bax and Bak proteins promote apoptosis by disrupting mitochondrial membrane integrity, leading to cytochrome c release and activation of the intrinsic apoptotic pathway.
Role of Anti-apoptotic Bcl2 & BclXL proteins
Bcl2 and BclXL proteins prevent apoptosis by inhibiting the activation of pro-apoptotic factors like Bax and Bak, thereby promoting cell survival.
In what position does phosphotidylinositol 3-kinase phosphorylate
3rd position
generates phosphoinositdes phosphorylated at the 3rd,4th, and 5th positions
would mutation of PI3K to a constitutively active kinase lead to more or less cell survival?
more cell survival
Would inactive PTEN ( phosphatase that converts PIP3 back to PIP2) lead to more or less cell survival?
more cell survival
What is the largest family of cell surface receptors ( in eukaryotic cells)
G protein-coupled receptors(GCPRs)
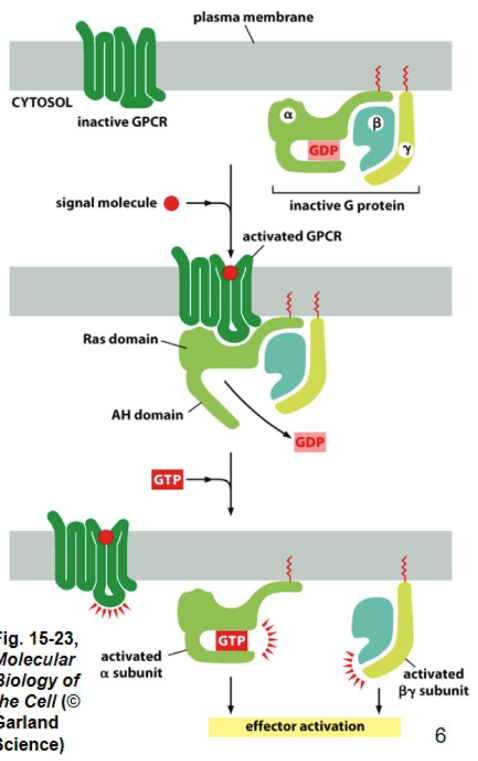
GCPRs
7 alpha-helical transmembrane domains
membrane domain binds signal molecule
domain → transduces signal
- anchored to inner leaflet (basically inside) of plasma membrane
Why are G-protein couppled receptors trimeric?
They consist of three subunits: alpha, beta, and gamma. This structure is essential for their role in signal transduction.
Alpha → GTP-binding domain
Beta & Gamma → control signaling
activation of a G protein by a GPCRs
DO NOT INTERACT WITHOUT OUTSIDE SIGNAL MOLECULES
1. g protein by to GDP
2. outside signal molecule binds to outside membrane part of GCPR ( embedded in membrane, half in each)
3. G ptotein attatches to GCPR
4. GCPR becomes a GEF ( guanine exchange factor) for G protein
4.5. GDP becomes GTP
5. GTP binds to alpha subunit of trimeric G protein
(beta and gamma subunits dissociate from alpha subunit, activating downstream signaling pathways.
(dissaciate → seperate from each other after change of GDP to GTP)
cyclic AMP (cAMP)
made from a pathway including type of trimeric G protein
second messenger involved in cellular signaling
that transmits signals from activated GPCRs to target proteins inside the cell.
cAMP example
synthesized from ATp by adenlyate cyclase
- second messenger
g proteins GS simulate cAMP production ( power it)
-( S = si)
- G proteins Gi inhibit camp productionI= inhibit
when signaling, CAMP phosphodiesterase attatches regular cAMP to AMP
how does cAMP affect cell behavior?
Signal molecule bind and activate coupled receptors (GCPRS)
GPCR activate alpha subunit(gtp-binding domain) of Gs( which turn on cAMP prdouction
Gs activates adenylate cyclase
adenylate cyclase convert ATP → cAMP
cAMP bind to regulating subunit of cAMP- dependent protein kinase A(PKA)
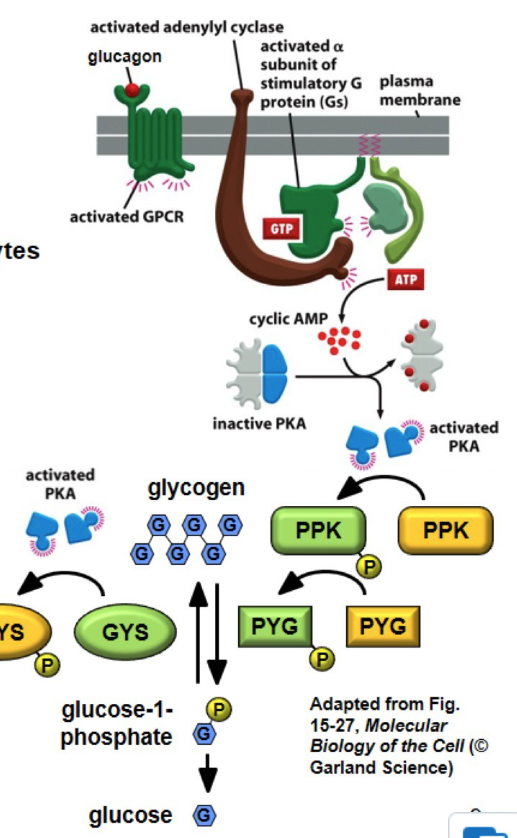
cAMP in glucose homeostasis
glucagon( released from pancreas when blood sugar is too low ; opposite of insulin) binds to a G protein coupled receptor in hepatocytes(liver cells)
GPCR signal Gs to activate PKA
PKA phosphorylates and activates PPK(phospohrylates → add extra P)
PPK phophorylates and activates PYG(glycogen phosphorylase)
PYG converts glycogen to glucose-1-phosphate and then made into glucose to combat low blood sugar
ALSO
PKA (made by cAMP) phophorylate and blocks glycogen synthase (GYS), preventing glucose to turn into glycogen, lowering blood sugar even further
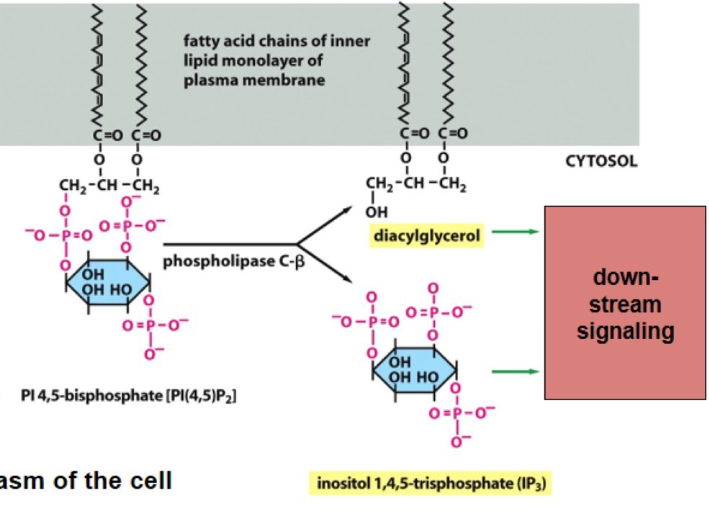
phospholipase C
Protein called Gq signals enzyme phospholipase C
after being turned on. PLC cleaves phosphatidylinositol 4,5-bisphosphate(PI(4,5)P2)
products are DAG and IP3 used for downsteam signaling
signaling through Gq change cell behavior?
Gq is activated by GCPR
Gq acltivates PLC
PLC activates DAG and IP3 by adding PI(4,5)P2
IP3 diffuses to the ER, and bind to a Calcium channel (Ca2+) that is IP3-gated
IP3 binding causes opening of Ca2+ and therefore release of Calcium into cytosol
Ca2+ binds to DAG and activates serine/threonine kinase protein kinase C
kinase C helps with smooth muscle contractions, vasodilation(dilation of blood vessels)
Calciums’(Ca2+) rolls in cell signaling
muscle contraction
neuromuscular junctions
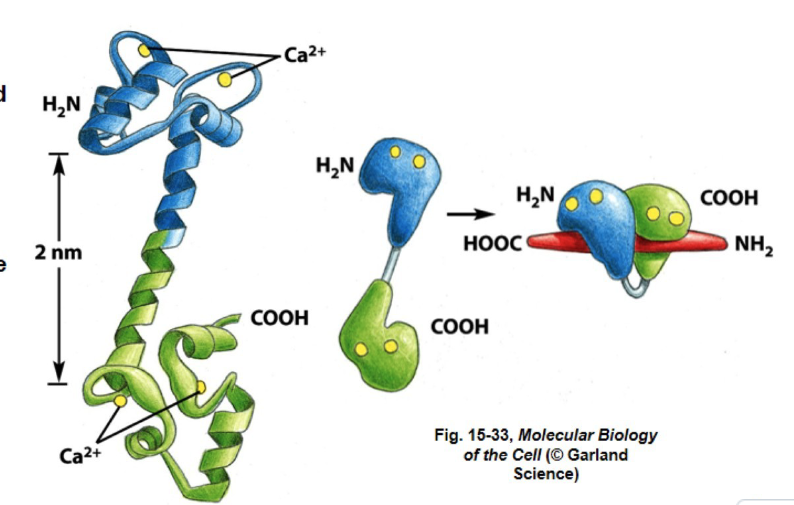
CALMODULIN
- two domains connected by an alpha helix
when calcium binds to calmodulin the protein changes its conformation and activates, regulating and binding with other proteins
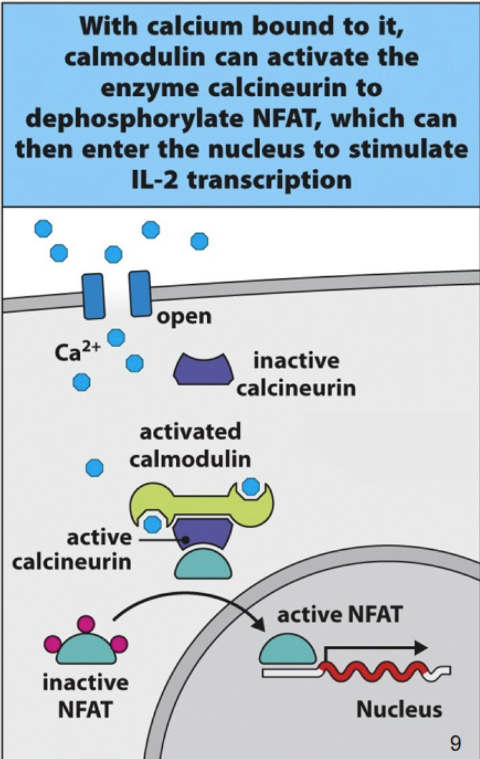
Ca2+ signaling in T Cell Activation
Ca2+ → IL-2
IL-2 regulator is a nuclear factor of activated t cells ( NFAT)
resting t cell = NFAT is phosphorylated and kept in cytoplasm
calmodulin and calnuerin are inactive
when a pathogen or antigen is recognized and it matches the one the t-cell is specific for phopholipase C is activated and Ca2+ enters cytoplasm
Ca2+ binds to calmodulin → binds to calcineruin → calcineurin activation
calcinuerin from NFAT
NFAT goes to nuclues to the IL-2 gene to begin cell division and attack
blocking t cells ( in the case of organ transplants or immunodefficiency issues)
CYCLOSPORIN A
made naturally by a fungus, discovered in the 80s
- enters a T cells and block signaling
- succesful methods for secure organ transplantation
mechanism for blocking t cell activation - cyclosporin A
cyclosporin A binds to immunophilin
- binds to calcineurin before calmodulin can → NFAT is still phosphorylated and inactive