Chapter 8 microbial metabolism slides
1/130
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
131 Terms
What is metabolism?
all chemical and physical workings of a cell
What are the two chemical reactions?
Catabolism and Anabolism
What is catabolism?
Degradative; breaks the bonds of larger molecules forming smaller molecules ; RELEASES ENERGY
What is anabolism?
biosynthesis; process that forms larger macromolecules from smaller molecules; requires energy input
What are enzymes?
Biological catalyst that increase the rate of chemical reactions by lowering energy needed
-Not permanently altered in reaction
-Promote a reaction by serving as physical site for specific substrate molecules to position
What are the different types of enzymes?
Simple enzymes
Conjugated enzymes or holoenzymes
Apoenzyme
Cofactors
What are simple enzymes
enzymes that consist of protein alone (Control mechanisms)
What are conjugated enzymes or holoenzymes?
contain protein and nonprotein molecules
What are apoenzymes?
protein portion of enzyme AND ARE THE ACTIVE SITE
What are cofactors in enzymes?
Nonprotein portion of enzyme
What do Apoenzymes do?
Exhibit primary, secondary and tertiary protein organization
(Larger enzymes show quaternary structure
What is the active site(catalytic site) ?
Site for specific substrate binding
What is an apoenzyme?
An enzyme without its cofactor.
What is the active site?
The region of an enzyme where substrate binds.
What is required for a union to occur in an enzyme?
There must be perfect fit between enzyme and substrate
What are metallic cofactors? (Metal ions)
The active enzymes
-Help bring the active site and substrate close together
-Participate directly in chemical reactions with the enzyme-substrate complex
What are coenzymes( organic factors)
-Serve as temporary carrier for some substrates
-Vitamins as the most common
What are exoenzymes?
transported extracellularly, where they break down large food molecules or harmful chemicals
-Cellulase, amylase and penicillnase
What are endoenzymes?
enzymes that function inside the cell; retained intracellularly and function in there
-Most enzymes are endoenzymes
What are constitutive enzymes?
always present, always produced in equal amounts or at equal rates, regardless of the amount of substrate (House keeping enzymes that aid in stuff like digestion)
What are regulated enzymes?
not constantly present; production is turned on (induced) or turned off (repressed) in response to changes in the substrate concentration
What is a condensation reaction?
Forming of glycosidic bonds between two glucose molecules to generate maltose. Requires the removal of water molecule!
What type of reaction is condensation reaction?
anabolic as it produces water as a product
What is a hydrolysis reaction?
Catabolic reaction that breaks down large substrates into smaller molecules
(Breaks peptide bonds between two amino acids)
-Requires the input of water to break bonds!
What influences the activity of an enzyme?
the cell's environment
What has to happen in order for an enzyme to operate?
There needs to constant and exact temperature, pH and osmotic pressure of organisms habitat
What makes enzymes change?
When habitat becomes unstable
What are labiles:
Chemically unstable enzymes
What is denaturation?
Weak bonds that maintain the shape of the apoenzyme are broken
What is the main difference between chemical reactions and biochemical reactions?
Chemical reactions occur instantly due to activation enzyme
Biochemical reactions occur like stairs and are slower
What is a Multienzyme system?
Metabolic pathways usually contain many steps each with an individual enzyme
SLIDE 24
What are the direct controls on the actions of enzymes?
Competitive inhibition
Allosteric inhibition
Noncompetitive inhibition
What is competitive inhibition?
substance that resembles the normal substrate competes with the substrate for the active site
What is allosteric inhibition?
Form of competitive inhibition in special types of enzymes with two binding sites; the active site and the regulatory or allosteric site
-Regulates enzymes via negative feedback from binding of molecules
Non competitive inhibition
Inhibitor binds to entire enzyme substrate complex and prevents the enzyme from completing its action on substrate
Methods of enzyme synthesis control
Enzyme repression
Enzyme induction
What is Enzyme repression? (Turns off enzyme)
automatic suppression of enzyme synthesis when end product builds to excess
-Long response time, but effects last longer
What is Enzyme induction? (Turns on enzyme)
enzymes are made only when suitable substrates are present
-Enables the organism to adapt to nutrients and prevents waste of energy
What is energy?
the capacity to do work or to cause change
What are the forms of energy?
Thermal, radiant, electrical, mechanical, atomic, andchemical
What are cell energenics
Cells manage energy in the form of chemical reactions tha make or break bonds and transfer electrons
What are the two forms of cell energenics
Endergonic reaction
Exergonic reactions
Endergonic reaction
A nonspontaneous chemical reaction, in which free energy is absorbed from the surroundings.
Consume energy
Energy +A + B ------(Enzyme)-----> C
exergonic reaction
A chemical reaction that releases energy
Release energy
X + Y ------(Enzyme)-----> Z+Energy
(Energy released is temporarily stored in high energyphosphate molecules. The energy of these molecules is usedin endergonic cell reactions.)
What are redox reactions?
chemical reactions that transfer electrons between reactants (Always occurs in pairs)
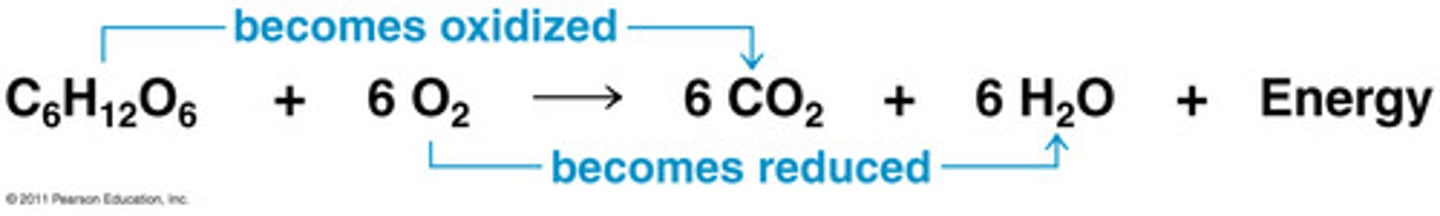
What are electron and proton carriers
-molecules that load and unload electrons and protons (H+)
Ex. NAD, FAD, FMN
Purpose of electron and proton carriers
Repeatedly accept and release electrons and hydrogen to facilitate the transfer of redox energy
Most carriers are coenzymes:• NAD, FAD, coenzyme A, and compounds of the respiratory chain
Oxidized AND Reduced example
NAD+ (OXIDIZED) NADH(Reduced)
What is ATP?
Metabolic "currency"
Three part molecule consisting of:•
Adenine - a nitrogenous base
• Ribose - a 5-carbon sugar
• 3 phosphate groups
What happens if terminal phosphate is released in ATP
Energy is released
Function of ATP
ATP utilization and replenishment is a constant cycle inactive cells
What is Phosphorylation of glucose by ATP
The metabolic process of introducing a phosphate group into glucose by ATP.
What kind of reaction is Phosphorylation of glucose by ATP
Anabolic
What are the three mechanisms of ATP synthesis?
Substrate-level phosphorylation
Oxidative phosphorylation
Photophosphorylation
What is substrate level phosphorylation?
Transfer of phosphate group from a phosphorylated compound directly to ATP
What is oxidative phosphorylation?
series of redox reactions occurring during respiratory pathway
What is photophosphorylation?
In photosynthetic organisms, utilize the energy of sunlight
What is bioenergetics?
study of the mechanisms of cellular energy release
Includes catabolic and anabolic reactions
Primary catabolism of fuels (glucose) proceeds through a series of three coupled pathways
examples of bioenergetics
Glycolysis
Krebs cycle
Respiratory cycle, or electron transport
Metabolic Strategies
aerobic respiration, anaerobic respiration, fermentation
Examples of Aerobic respiration
Respiration that requires oxygen; glycolysis, the Kreb's cycle,respiratory chain
Examples of Anaerobic respiration
Respiration that does not require oxygen; Glycolysis, the Kreb's cycle, respiratory chain; molecular oxygen is not the final electron acceptor
Examples of Fermentation
What is Aerobic respiration
Series or enzyme-catalyzed reactions in which electrons are transferred from fuel molecules to oxygen as a final electron acceptor
What are cycles that enable Aerobic respiration
Glycolysis
TCA
Electron transport chain
What is glycolysis?
the breakdown of glucose by enzymes, releasing energy and pyruvic acid.
What is the Krebs cycle (TCA)?
second stage of cellular respiration, in which pyruvic acid is broken down into carbon dioxide in a series of energy-extracting reactions
What is the electron transport chain?
accepts electrons from NADH and FADH; generates energy through sequential redox reactions called oxidative phosphorylation
What is the input of glycolysis?
1 glucose, 2 ATP
What is the output of glycolysis?
2 pyruvate, 2 ATP, 2 NADH
What is the input of the Krebs cycle?
2 Acetyl CoA
What is the output of Krebs cycle
4 CO2, 6 NADH, 2 FADH2, 2 ATP
What is the input of the electron transport chain?
NADH and FADH2
What is the output of the electron transport chain
H2O and ATP (02 is the final electron acceptor)
What is the energy investment stage
the cell uses ATP to phosphorylate compounds of glucose
How much ATP is used in the investment stage
TWO ATP MOLECULES PER STAGE
Gross ATP glycolysis
4 ATP
Net ATP of glycolysis
4 produced - 2 invested = 2 net ATP
what fuels the electron transport chain?
H+
What is the chain of redox carriers in electron transport?
Chain that receives electrons from reduced carriers (NADH and FADH2).
What is the role of ETS in electron transport?
Shuttles electrons down the chain, releasing energy that is captured and used by ATP synthase complexes to produce ATP.
What is the function of the periplasmic space
Folding proteins and storage of protons
The Terminal Step
Oxygen accepts 2 electrons from the ETS and then picks up 2 hydrogen ions from the solution to form a molecule of water. Oxygen is the final electron acceptor
How much ATP is produced in aerobic metabolism
34 ATP
Glycolysis NET SUMMARY
2ATP
2NADHs
2 Pyruvic acids
Transition step NET SUMMARY
2NADHs
2 CO2S
Krebs cycle NET SUMMARY
4 CO2s
6NADHs
2 ATPS
2 FADH2S
Elective transport and oxidative phosphorylation NET SUMMARY
34 ATP
6 H20S
What is anaerobic respiration?
Functions like aerobic respiration except it ultizes oxygen containing ions, rather than free oxygen as the final electron acceptor
Nitrate (NO -3) and Nitrate (NO -2)
What do most obligate anaerobes use?
H+ generated during glycolysis and the krebs cycle to reduce some compound other than O2
What is nitrification?
a process that breaks down ammonia into nitrites or nitrates
Ammonium---->Nitrate
Ammonia nitrication
Ammonium (NH4+) --(Oxidized)----> Nitrate (NO2-)----->(Oxidized)----------> Nitrate (NO3_)
Denitrification (nitrogen cycle)
This is the reverse process of nitrification. During it nitrates are reduced to nitrites and then to nitrogen gas and ammonia.
Nitrate-----> Ammonia
What is fermentation?
Incomplete oxidation of glucose or other carbohydrates in the absence of oxygen
What does fermentation use?
Uses organic compounds as terminal electron acceptors to yield a small amount of ATP
outcome of fermentation
Ethyl alcohol by yeast acting on glucose and formation of acid ,gas and other products by the actions of various bacteria on pyruvic acid
Purpose of fermentation for bacteria
To regenerate NAD+ (Nicotinamide adenine dinucleotide)
What is the advantage of fermentation? (EXAM QUESTION)
To generate ATP
Pathways involved in aerobic respiration
Glycolysis, Krebs Cycle, ETC
GARFIELD
KILLS
ELFS