SNC1W1 Chemistry: Atoms, Elements and Compounds
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms

Biohazardous, Infectious materials - for organisms/toxins that can cause diseases in people/animals
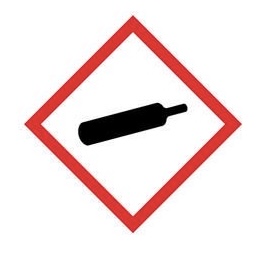
Compressed gas - for gases under pressure
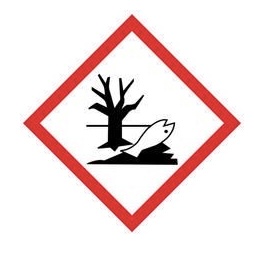
Environmental hazard - potential damage to the environment

Acute Toxicity - can cause death/toxicity with short exposure to small amounts.

Exclamation mark - can cause irritation to skin and eyes

Flammable - for fire hazards
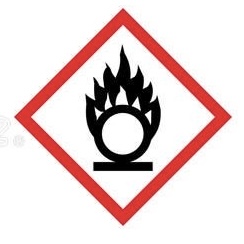
Oxidizer - for oxidizing hazards
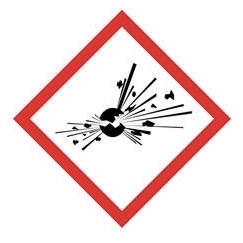
Explosive - for explosion or reactivity hazards
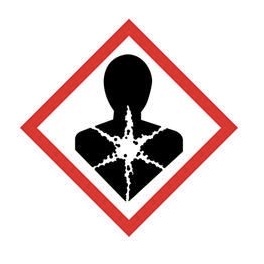
Health Hazard - may cause/suspected of serious health effects
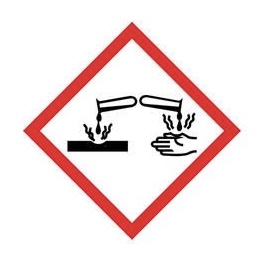
Corrosive - for corrosive damage to metals, eyes and skin.
How can you determine the difference between a WHMIS symbol and a HHPS symbol?
A WHMIS symbol is typically in a circle, while an HHPS symbol is in either an octagon, diamond or rounded-triangle.
WHMIS
Workplace Hazardous Materials Information System.
MSDS
Material Safety Data Sheets
HHPS
International Hazard Symbols
Octagon HHPS Symbol
Danger
Diamond HHPS Symbol
Warning
Triangle HHPS Symbol
Caution
The Particle Theory of Matter
All matter is made up of tiny particles.
Each pure substance has its own kind of particle, which is different from the particles of other pure substances.
Particles attract each other.
Particles are always moving.
Particles at a higher temperature move than particles at a lower temperature.
Pure substance
matter that contains only one kind of particle
Mixture
matter that contains more than one kind of particle
Two main groups of pure substances
elements and compounds
Element
pure substance that can’t be broken down into simpler parts by chemical methods
compound
pure substance made of two or more different elements that are chemically combined
Physical property
a trait of a substance that can be watched and measured without changing the identity of the substance.
Qualitative physical property
can be observed and described without measurement. ex. colour.
Quantitative physical property
can be measured and assigned a particular value. ex. melting point of a compound.
Solubility
a measure of the ability of a substance to dissolve in another substace.
Hardness
a substance’s ability to be scratched
Density
the ratio of the mass of a substance to the volume it occupies
Density formula
m/v
Chemical propertiies
the ability of a substance to change/react and form new substances
Combustibility
the ability of a substance to burn in air
Stability
the ability of a substance to remain unchanged
Toxicity
the ability of a substance to cause harmful effects in nature