Isomerisation and intermolecular forces
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
What is isomerism?
The existence of molecules with the same molecular formula but different structural formulas or spatial arrangements of atoms.
same number and type of atoms
Structural- Chain isomerism
different arrangement of a molecules carbon skeleton
Creates branched carbon chains off the main chain
Makes them more compact = lower boiling point and higher melting point
Same molecular formula
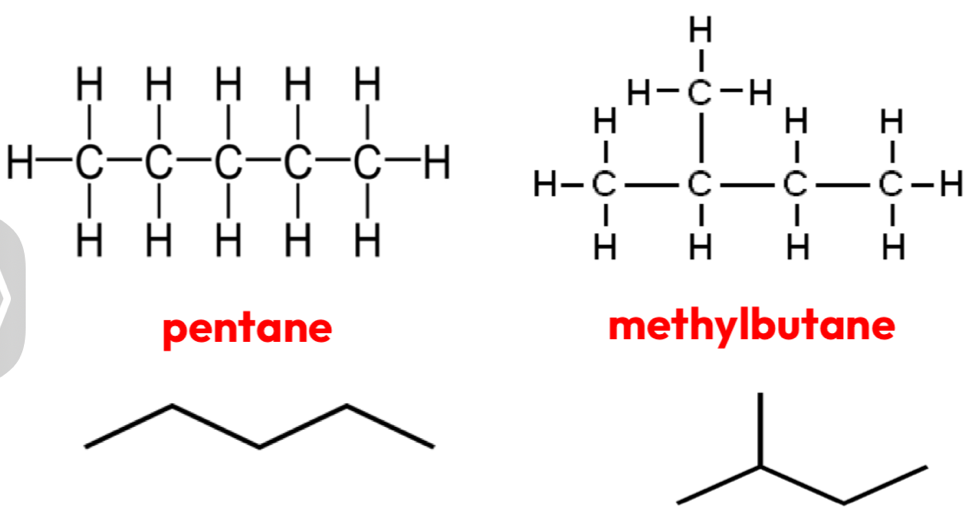
Structural- Position isomerism
Differing position of the same functional group in a molecule
The name of the molecule changes to reflect its new position
Same molecular formula
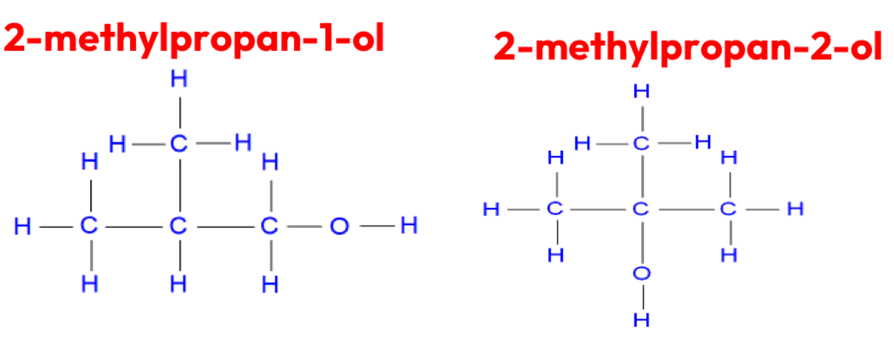
Structural- Functional group isomerism
Isomers have the same molecular formula but different functional groups
Differing positions of atoms (rearranged)
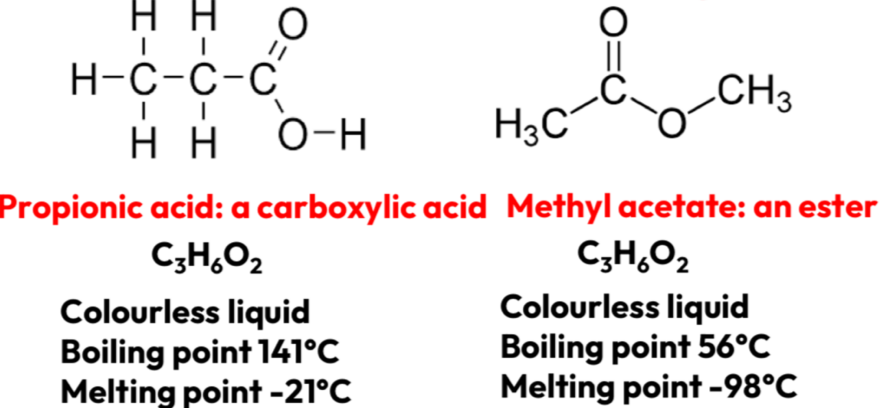
Sterioisomerism
Where molecules have the same molecular formula but different structural formulas in 3D orientations of their atoms.
Stero- Geometric
the substituents around a bond with restricted rotation are different
Two types: cis and trans
Usually around a restricted bond e.g.C double bond
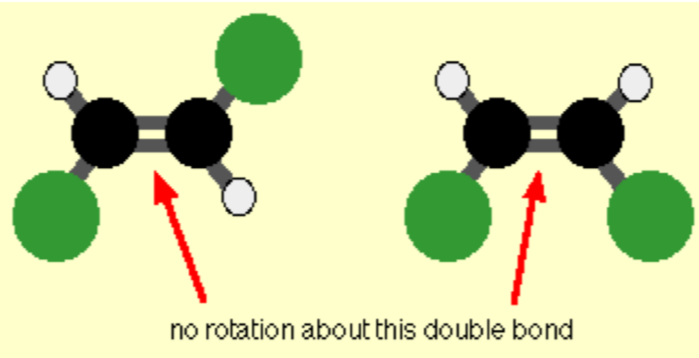
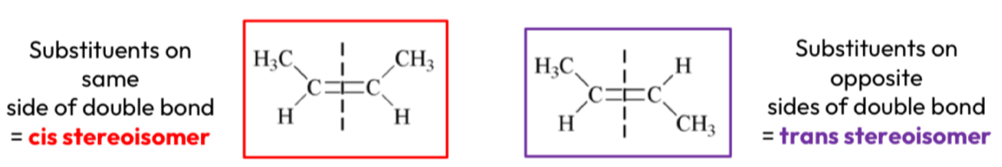
What are cis/trans stereoisomers?
Cis stereoisomers = substituents on the same side of the double bond
Trans stereoisomers = substituents on opposite sides of the double bond
Stereo- Optical
non-superimposable mirror images of the same molecule
Cannot be perfectly aligned onto another
Identical physical properties except interaction with plane-polarised light
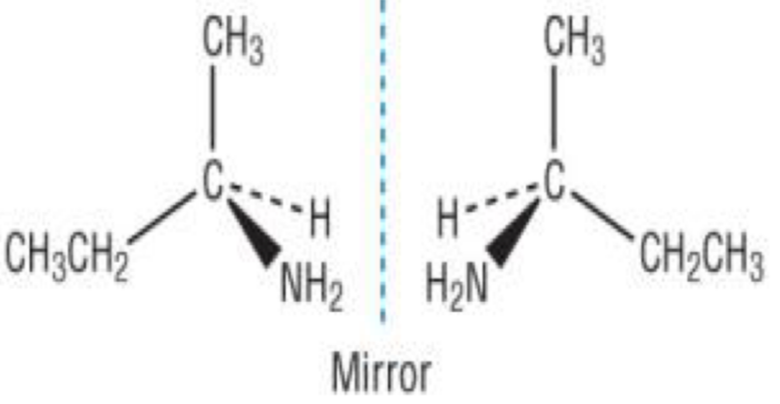
Enantiomers
Stereoisomers
Differ in arrangement at positions called chiral centres (central carbon)
Must have at least one chiral centre
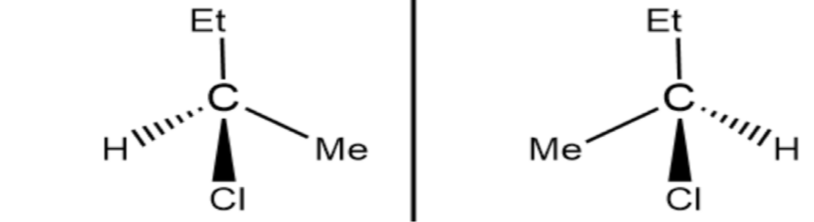
Chiral centres
Central carbon atom bonded to 4 groups
Cannot be bonded to two+ of the same group E.g. 2H
Cannot be a double bond
Cannot have any symmetry
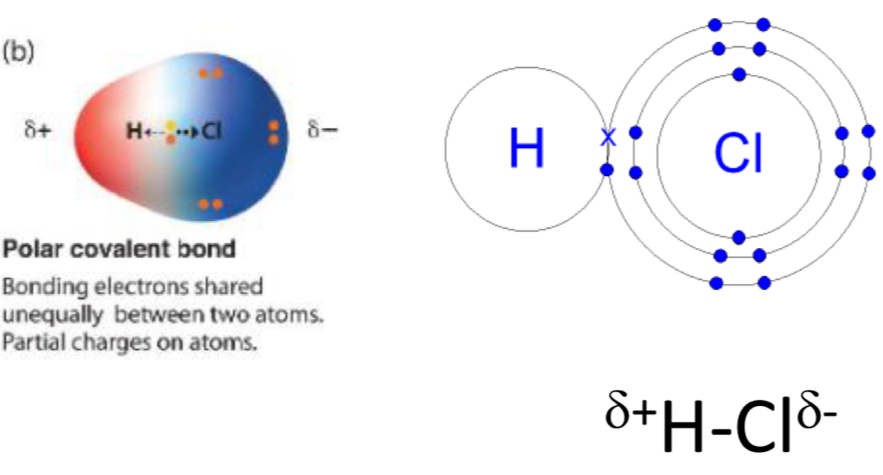
Dipoles in polar bonds
a polar covalent bond has a permanent dipole (slight -ve charge and slight +ve)
Dipole = molecule whose ends have opposite charges e.g. HCl
H2O is a polar molecule, with a dipole +ve at the H2 end and -ve at the O end
Polar bonds may not have a Dipole e.g. CCl4 (-ve charges spread out around C so NO NET DIPOLE)
Medium strength, stronger when molecules are closer together
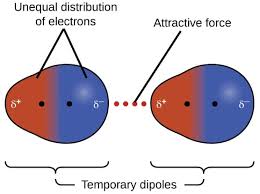
London dispersion/Vander Walls
attraction between 2 instantaneous dipoles
Asymmetrical electron distribution possible at any point
All atoms and molecules
Weakest intermolecular force
Increase in strength as molar mass increases
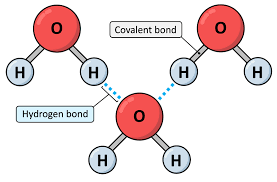
Hydrogen bonding
attraction between molecules with NH, OH or FH bonds
Extremely polar bonds = very strong dipole-dipole forces
H atom is attached to an electronegative atom, but also attracted to another molecules electronegative atom
E.g. water
Strongest intermolecular force
How do polar solvents interact?
WITH A POLAR SOLUTE
e.g. water H2O
Hydrogen bonding occurs (attraction to -ve atoms) so the solution is mixed
WITH A NONPOLAR SOLUTE
e.g. hexane and water
Solute and solvent stay separate