Quantum Theory - Light as Wave & Particle
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
if we cant see or touch the atom, how do we study it
atoms respond like waves do (react to elec and magentism) so we use wave properties to study them
what is the theory of light as a wave called + by who and what it states
james maxwell proposed the classical theory of light
- ligt exists as an EM wave (made of electric and magentci feilds) and has elec and magentic properties
- so light is a continuous wave of energy (no gaps breaks, just directly from the source)

recall wavelength vs freq vs speed of light
- wavelength = lambda > distance from crest to crest/trough to trough
- frequency (f or sometimes v (greek nu) - the number of complete wavelengts (cycles) that pass a given point each second (units: s^-1 or /s >>> or Hertz Hz
- speed of light (3.00x10^8 m/s >> Alpha C on calc)
em spectrum wavelength frequency trend and which ones are more dangerous
from L to R, as wavelengths descrease, freuency increases (inversely proprtional)
- long wavelength, shorter frequency = low E > less danger (radio tv waves)
- short wavelength, high f = high E radiation > danger more (gamma)
eqn for frequncy
f = c/λ
3 things that the wave model of light couldnt explain
1. the emission of light from hot objects (blackbody radiation)
2. the emission of e-'s from metal surfaces on which light shines (photoelectric effect)
3. the emission of light from electronically excited gas atoms (emission spectra)
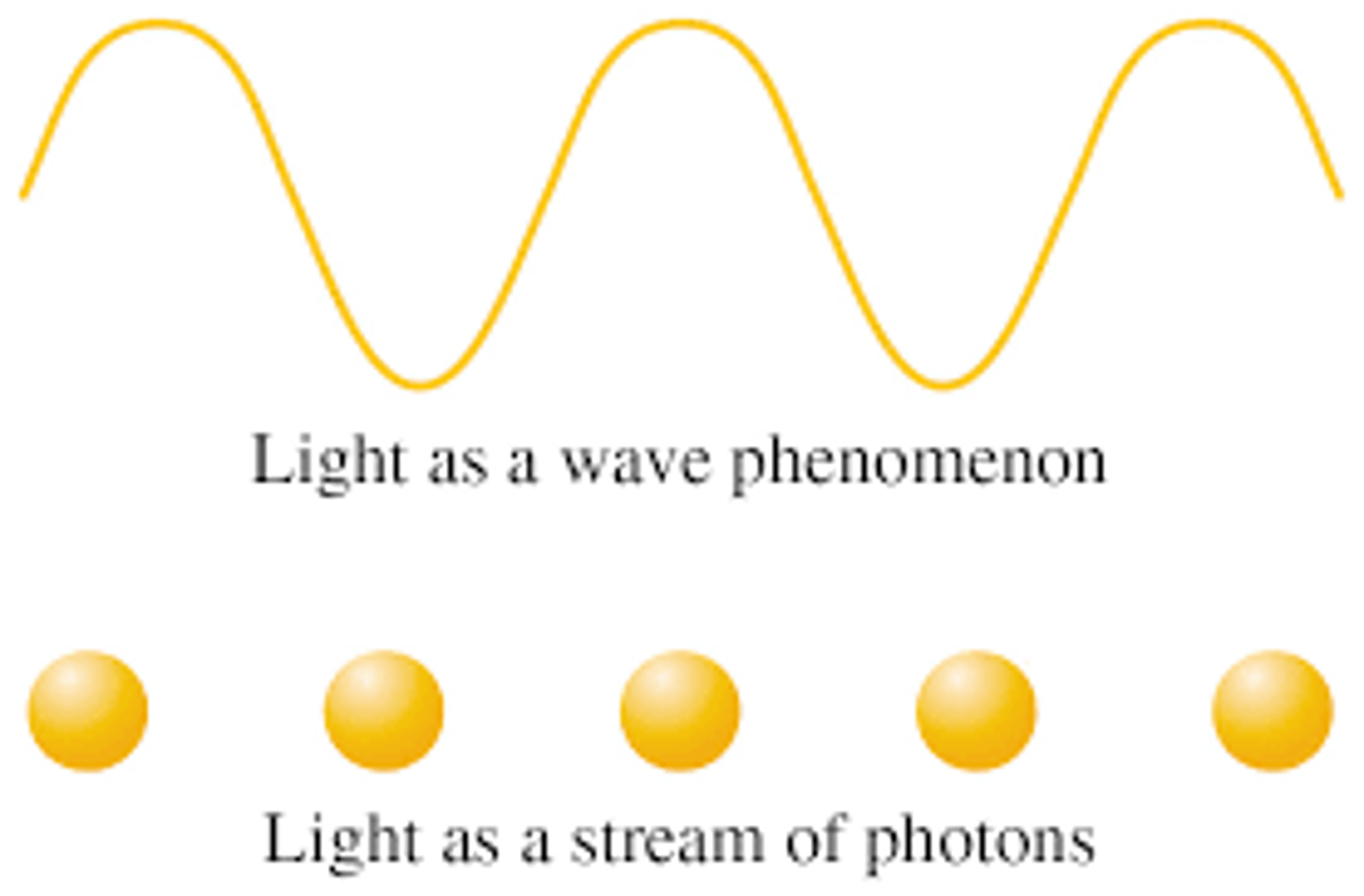
what was the 2nd theory abt light + who beleived/contributed to it & what to note
ancient greek philosphers + isaac newton (17th century believed light was a stream of tiny individual particles
^ newton called these corpuscles
note - if true, why cant we see the gaps bw the tiny particles? >> too many inividual particles + travelling at speed of light so we dont see the gaps in bw
what discovery led to the rise of this theory and when and by who
1887 - heinrich hertz discovered the photoelectric effect - the emission of e-'s from metal surfaces on which light shines
^ this is one of the things that the wave model of light couldnt explain
describe the process of the photoelectric effect, the hypothesis, and the reality >>> + what theory arose as a result
when light was shone on an obj, the light particles collide with the surface of that obj > gives E to the object which releases e-'s that land on a detector creating current
- hypothesis (according to wave theory) was that as the shining light became brighter (greater intensity of wave), it should impart more E to the e-'s when the force feild collides > they should have more kinetic E so more e-'s should emit from the obj
- BUT that didnt happen. >> it was acc the frequency of the light (ie its colour and thus its energy) that determined the kinetic E of the e- released from the obj
^^unable to be explained by hertz
>> led to quantum theory
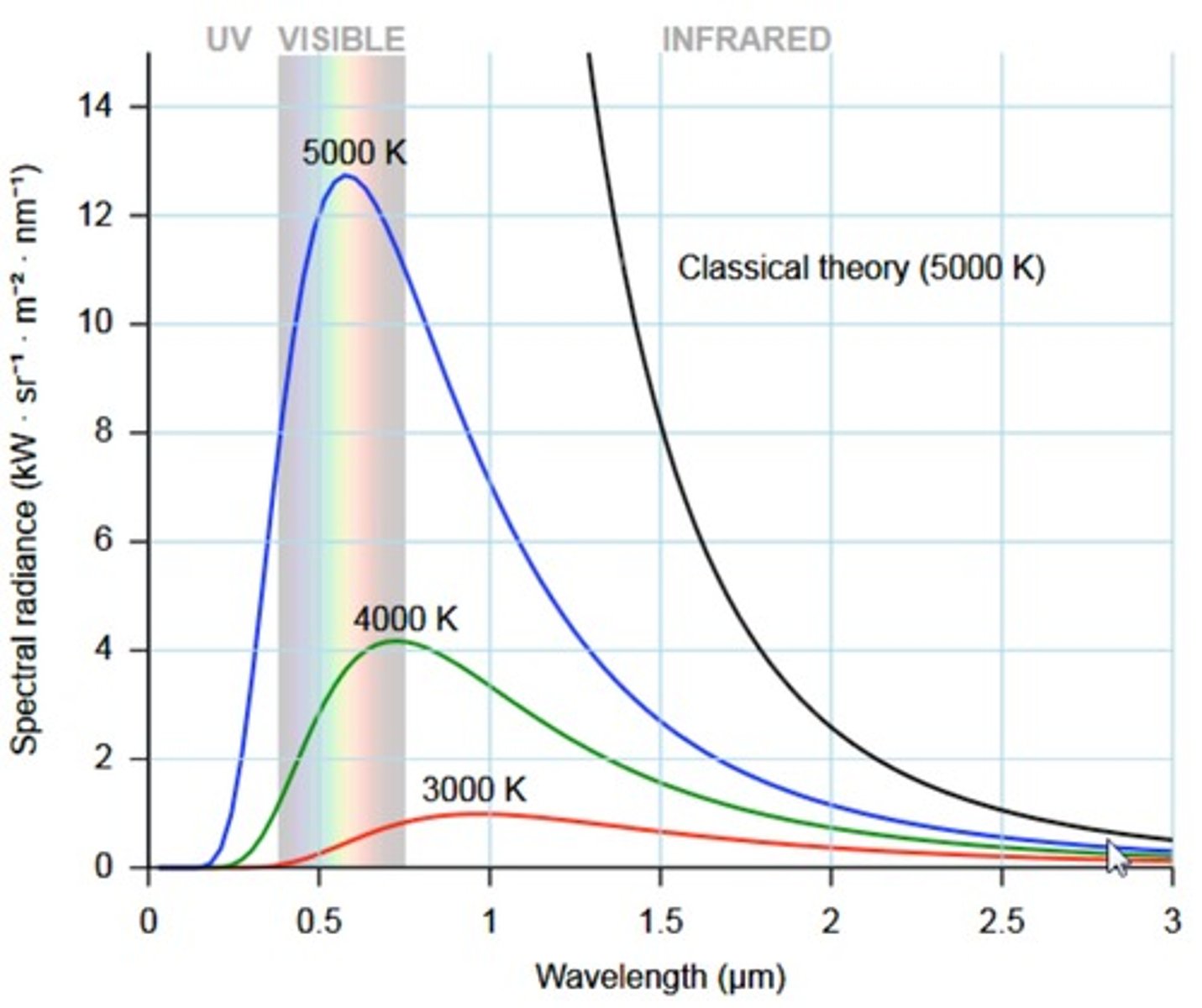
describe the UV catastrophe and why it didnt align with the wave theory
- when solids heated > emit radiation
blackbodies are radiating objs
- eqn created to predict blackbody radiation which stated that the higher the f, (short wavelength) > the hgiher the intensity
- btu when f got into UV range > measuremnts didnt make sense >> at acertain freq, light would hit peak intensity and then intensity would drop as freq increased
^^^aka the catastrophe - bcs as frequency got higher, intensity was supposeed to keep getting stronger (according to wave theory)