Chemistry 2.6 + 2.4
1/32
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
What are acids?
Proton donors (+, usually)
What are bases?
Proton receptors (-, usually)
What are conjugate pairs?
Acid/base combo that differs between 1 proton. Can switch between acid/base.
Strong acid/base
Fully dissociates in water. Acid produces lots of H3O+ ions, base produces lots of OH- ions.
Weak acid/base
Partially dissociates in water. Creates a reversible, two-way reaction which forms an equilibrium.
Acid
pH less than 7
Base
pH greater than 7
Neutral
pH equal to 7
Current
Flow of charge. Flow of charge is due to charged ions, which creates a current.
Conductivity
Conductivity depends on the number of mobile charged particles (can be ions). Strong acids/bases will be more conductive, as they fully dissociate in water producing ions. The amount of charged ions in a solution determines the electrical conductivity of a given solution.
Amphiprotic
A substance that can accept and donate protons e.g. water.
What do acids and bases produce in a solution?
Acids produce hydronium ions (H3O+), bases produce hydroxide ions (OH-) in a solution.
When is a two way reaction at equilibrium?
When the forward and reverse reaction is happening at the same rate. Overall effect = we cannot see anything happen as they cancel out.
What does equilibrium not mean?
Equilibrium does not mean the amount of reactants is equal to the amount of products. It also does not mean that the reaction has stopped.
Le Chatelier’s principle
"Any change made to a system in equilibrium results in a shift of the equilibrium in the direction that minimises the charge.”
Catalysts
Catalysts lower the energy required for the reaction to take place (activation energy) + has no effect on equilibrium position.
Pressure
Increase in pressure favours products, decrease in pressure favours reactants (weak acid/base)
Concentration
If more products are added, the system will ‘add’ more reactants by favouring reactant → product equation. If more reactants are added, the reverse will happen.
Temperature
Exothermic = energy given out to surroundings (change in enthalpy = -). Endothermic = energy taken in from surroundings (change in enthalpy = +).
Temperature and equilibrium
In an equilibrium system, one reaction will be exothermic and the other will be endothermic. In the endothermic reaction, products are favored over the reactants. In an exothermic reaction, the reactants are favoured over the products.
Temperature and Kc
Endothermic causes Kc to increase, exothermic causes Kc to decrease.
Kc
The mathematical representation of the position of the equilibrium, which requires no units. It compares the concentration of products and reactants. Kc = [products] divided by [reactants]
What does Kc not include?
Solids and liquids, as they are constant.
What does Kc include?
Gases and aqueous substances
Collision theory
Reactions involve a transformation, where the reactant particles (atoms or molecules) combine to form products. Particles in a reaction are randomly moving and can be altered with multiple factors.
Activation energy
The minimum amount of energy required for a chemical reaction to occur between reactant particles.
Concentration
Increase in concentration will increase the frequency of collisions, increasing the reaction rate.
Surface area
An increased surface area will increase the reaction rate, as the surface layers are the only sides a particle can react with to produce products. increased frequency of collisions = increased successful collisions = increased rate of reaction.
Kinetic energy (form of temperature)
increase in temp → particles gain kinetic energy → frequency of collisions increase → increased successful collisions → increased rate of reaction
Activation energy (form of temperature)
Temp provides the particles the minimum amount of heat energy required. Increase in temp → reaction happens sooner → rate of collisions increase → rate of reactions increase. Decrease in temp → reaction happens less → less collisions → rate of reaction slows.
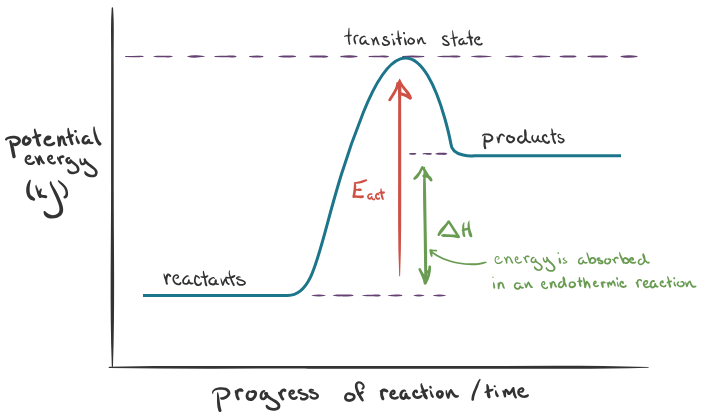
Endothermic graph
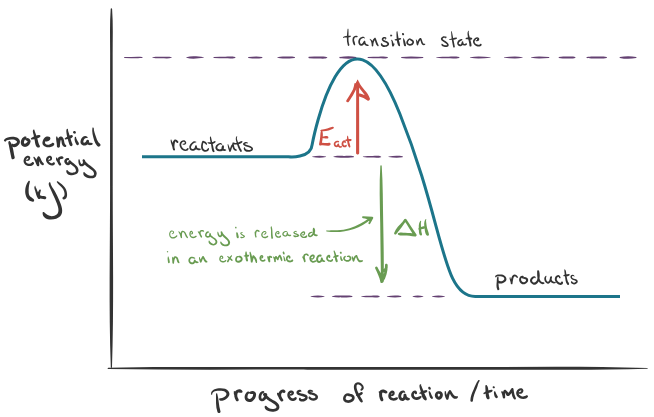
Exothermic graph
Enthalpy calculations
Energy (kJ) = change in enthalpy (kJ mol-1) x n (number of moles)