Physical properties of alcohols and ethers
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
General definition of bp
Boiling point is a function of secondary attractive forces.
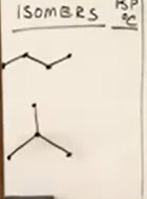
Review isomers boiling point effected.
The isomer with the higher boiling point is the straight chain. This is because as you increase branching, you reduce secondary attractive interactions.
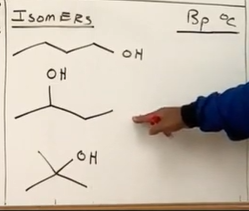
Which has the highest boiling point of these three alcohols?
The top option has the higher boiling point because less branching. The more branching the less secondary attractive forces.
Relationship between molecular weight and bp
Generally as mw increases, bp increases.
Asymetrical ether bp vs symmetrical
Asymmetrical ethers are more polar. The more polar something is, the boiling point increases because involves dipole dipoles or h bonding interaction.
boiling point alcohols vs ethers
alcohols have much higher boiling points because hydrogen bonding in the H—O bond. Strong interactions. Alcohols can form hydrogen bonding with themselves while ethers cannot.
Are alcohols and ethers water soluble?
Yes both are. This is because hydrogen bonding forms between hydrogen and the oxygens of the alcohols and ethers.
Solubility of ROH RH and ROR in water
RH is zero, hydrocarbons are non polar and not soluble.
ROR is a much more soluble than RH. and ROH is slightly more soluble than ROR. Both more soluble than RH bc hydrogen bonding in water.
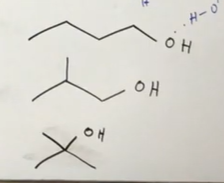
Solubility of these three alcohols
The straighter the chain, the LOWER the solubility. For isomeric alcohols, as you increase branching, you increase solubility. This is because when you do that, you make this “less” alkane like, and we know alkanes are not soluble.