BIEN135 ch6-9 MC HW Questions
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
True or False. A piston containing an ideal gas expands isothermally from 7 atm pressure to 2 atm pressure. The energy of the system (that is, the contents of the piston) remains constant during this process
True
A protein is negatively charged, but it binds a negatively charged small molecule faster than it binds a positively charged small molecule. The most reasonable explanation for this phenomenon is:
a. even though the protein is negatively charged overall, electrostatic focusing effects provide a pathway for a negatively charged molecule to enter the active site
b. the presence of water molecules screen the electrostatic effects
c. the negatively charged molecule makes stronger hydrogen bonds than the positively charged molecule
d. charged proteins normally bind substrates with the same overall charge
even though the protein is negatively charged overall, electrostatic focusing effects provide a pathway for a negatively charged molecule to enter the active site
The enthalpy change for a process is equal to the heat transferred to the system under which of the following conditions?
a. constant volume
b. constant pressure
c. constant temperature
d. reversible expansion
e. expansion at a constant pressure followed by reversible compression
constant pressure
Which of the following properties are extensive (choose all that apply)
temperature, pressure, amount of heat released, density, energy, molarity, number of moles, mass, volume
amount of heat released, energy, number of moles, mass, volume
True or False. Energy is a good indicator of the direction of spontaneous change for macroscopic mechanical objects but not for molecular processes.
True
Which of the following statements about potential energy is true?
a. the potential energy of an ideal gas is equal to its kinetic energy
b. the potential energy of an atom is the work done in moving the atom from infinity to its present position
c. potential energy is always absolutely conserved
d. the potential energy of a system always incerases
e. potential energy is an intensive function
the potential energy of an atom is the work done in moving the atom from infinity to its present position
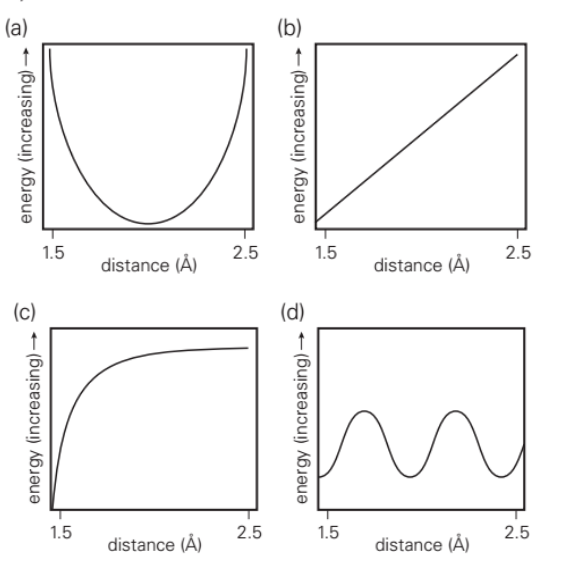
The type of function that best describes the energy of a hydrogen bond as the distance between the hydrogen atom and the acceptor atom varies between 1.5 and 2.5 A is: a, b, c, or d
a
A reaction that releases energy is called an _____ reaction
exothermic
Electrostatic interactions are governed by ______’s law
Coulomb
True or False. It is easier to predict the bulk behavior of a small number of molecules than a large number of molecules.
False
True or False. Consider a coin with two sides (H=heads; T=tails). The probability of observing HHHHHTTTTT is equal to the probability of observing HTHTHTHTHT.
true
True or False. An isolated molecular system exists in two states of equal energy, State A has high multiplicity, whereas State B has low multiplicity. Without any external agents a system in State B will spontaneously convert to State A.
true
True or False. A state corresponds to many different microsates
true
The work done in a near-equlibrium expansion of an ideal gas is _____ than for a nonequilibrium expansion.
greater
When the volume of a system increases its multiplicity_____
increases
True or False. For the system converting from state 1 to state 2: (kBlnW2-kBlnW1)=qrev/T
true
True or False. If the second lowest energy level is separated from the ground state by 0.5kBT, then the second lowest energy level will not be occupied appreciably
false
Which of the following definitions of etnropy are equivalent for a large system:
a. probabilistic definition
b. thermodynamic definition
c. statistical definition
d. all of the above
all of the above
After spontaneous heat transfer between systems, the overall multiplicity is:
a. lower than it was before the transfer of heat
b. zero
c. maximized
d. minimized
maximized
The Boltzmann distribution describes the energy of molecules at _____
equilibrium
The Boltzmann constant is the gas constant (R) divided by ________
Avagadro’s number
Kinetic energy is due to the ______ of atoms. Potential energy is due to the _______ of atoms.
motion, configuration/relative positions
The direction of spontaneous change is governed by an increase in ________, thus explaining how energy can be transferred “uphill”, that is from a system of lower total energy to one with higher total energy
entropy/multiplicity
True or False. For a system at equilibrium, Gibbs free energy is maximized
false
The work done by biological systems is most commonly which type of work?
a. pressure
b. chemical
c. home
d. volume
e. heat
chemical
According to Le Chatelier’s principle?
a. a reaction always favors the formation of as much product as possible
b. reactants will only react upon the addition of external heat or pressure
c. the system will adjust, if more reactants are added, and a new equilibrium will be established to balance the change
d. the Gibbs free energy is always greater than the amount of work done plus the Helmholtz free energy
the system will adjust, if more reactants are added, and a new equilibrium will be established to balance the change
True or False. The cell generates ATP from ADP and Pi by coupling the synthesis to an energetically unfavorable process
false
At equilibrium:
a. there is no change in temperature over time
b. the free energy of the system is minimized
c. the combined entropy of the system and surroundings is maximized
d. only b and c
e. a, b, and c
a, b, and c
Gibbs free energy is used to describe systems with constant ______, while Helmholtz free energy is used to describe systems with constant _____
pressure and temperature, volume and temperature
The change in free energy of a process is equal to the ______ amount of work extracted from that process
maximum