HPS2 CH 11 SG
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
What is the best way to really prove to yourself that you understand an idea?
If you can adequately explain this idea to others, then you have passed a high standard test of understanding.
Is chemistry the study of the submicroscopic, the microscopic, the macroscopic, or all three? Defend your answer.
Chemistry is the careful study of matter and can take place at a number of different levels including the submicroscopic, microscopic, or macroscopic levels.
You combine 50 mL of water with 50 mL of purified alcohol and get a total of 98 mL of mixture. Explain.
The 50 mL plus 50 mL do not add up to 100 mL because within the mix, many of the smaller water molecules are able to fit within the pockets of space that were empty within the 50 mL of larger ethanol molecules. This is analogous to the exercise involving the BBs and is yet another example where the existence of molecules helps to explain observed phenomena.
Which has stronger attractions among its submicroscopic particles at 25°C: a solid or a gas? Explain.
At 25°C, there is a certain amount of thermal energy available to all the submicroscopic particles of a material. If the attractions between the particles are not strong enough, the particles may separate from each other to form a gaseous phase. If the attractions are strong, however, the particles may be held together in the solid phase. We can assume, therefore, that the attractions among the submicroscopic particles of a material in its solid phase at 25°C are stronger than they are within a material that is a gas at this temperature.
Is it possible for air to be in the liquid phase? Please explain.
At sufficiently cold temperatures, the molecules of the air slow down enough to hold together in the liquid phase. This temperature is about -200 degrees celcius , which is not something we normally enounter here on Earth. Using powerful refrigerators and pumps, however, we can create these ultra cold temperatures to condense the air into a liquid phase.
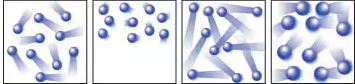
The leftmost diagram below shows the moving particles of a gas within a rigid container. Which of the three boxes on the right—(a), (b), or (c)—best represents this material upon the addition of heat? (Pictures are reversed in answer)
(b) The trail lines behind each circle in this diagram are meant to indicate that the particles are moving faster. Diagram (a) shows the particles congregated to one side of the box. This is an unlikely scenario because the motion of gaseous particles are randomly oriented. You may have interpreted diagram (a) to mean that the gas was hotter, and as every one knows, hot air rises. Fair enough, but notice how the trail lines in this diagram do not indicate any faster motion. Diagram (c) shows fatter gas particles. Upon the addition of heat, gas particles don’t grow any fatter. Instead, they move faster, which is to say that they have a greater average kinetic energy.
A cotton ball is dipped in alcohol and wiped across a tabletop. Explain what happens to the alcohol molecules deposited on the tabletop? Is this a physical or chemical change?
The molecules of the alcohol evaporate into the gaseous phase, which is a physical change.
A cotton ball dipped in alcohol is wiped across a tabletop. Would the resulting smell of the alcohol be more or less noticeable if the tabletop were much warmer? Explain.
The greater warmth of the table top provides more energy for the alcohol molecules to go into the gaseous phase. The rate at which the alcohol evaporates would be greater. As a result, there would be more alcohol molecules in the air more quickly, which would register in your nose as more noticeable.
Alcohol wiped across a tabletop rapidly disappears. What happens to the temperature of the tabletop? Why?
As the alcohol evaporates, it soaks up energy from the table top, which is thus cooled. This transfer of energy that occurs during a change in phase was discussed in Chapter 6.
Why does perfume produce a stronger scent when placed over a warmer spot on the skin, such as over the jugular vein on one’s neck?
Perfume is only odoriferous as it transforms into the gaseous phase in which it can travel through the air to one’s nose. Greater warmth on the skin provides more energy, which is needed to allow a phase change from liquid to gas.
In winter, Vermonters make a tasty treat called “sugar on snow,” in which they pour boiled-down maple syrup onto a scoop of clean fresh snow. As the syrup hits the snow it forms a delicious taffy. Identify the physical changes involved in the making of sugar on snow. Identify any chemical changes.
Boiling down the maple syrup involves the evaporation of water. As the syrup hits the snow it warms the snow, causing it to melt, while the syrup becomes more viscous. These are all examples of physical changes. Interestingly, as the maple syrup is boiled, the sugar within the syrup begins to caramelize, which is an example of a chemical change.
Each night you measure your height just before going to bed. When you arise each morning, you measure your height again and consistently find that you are 1 inch taller than you were the night before but only as tall as you were 24 hours ago! Is what happens to your body in this instance best described as a physical change or a chemical change? Be sure to try this activity if you haven’t already.
That this process is so reversible suggests a physical change. As you sleep in a reclined position, pressure is taken off of the discs within your spinal column, which allows them to expand so that you are significantly taller in the morning. Astronauts returning from extended space visits may be up to two inches taller upon their return.
State whether each of the following is an example of a physical or chemical property of matter:
Carbon dioxide escapes upon the opening of a soda can.
A bronze statue turns green.
A silver spoon tarnishes.
Physical property: a; Chemical property: b, c.
Why is the air over a campfire always moist?
As a tree grows, it uses the energy of sunlight to convert carbon dioxide and water into molecules of glucose, which is a carbohydrate. This process is called photosynthesis. The glucose molecules then link together to form cellulose, which is a main ingredient of wood. As wood burns in a campfire, the energy captured by photosynthesis is released back into the environment along with carbon dioxide and water. The heat of the campfire transforms the water into water vapor, which rises with the flames. Hold your hand high over a campfire and you will be able to feel humidity created by this escaping water vapor.
Classify the following changes as physical or chemical. Even if you are incorrect in your assessment, you should be able to defend why you chose as you did.
Grass grows.
An infant gains 10 pounds.
A rock is crushed to powder.
A tire is inflated with air.
chemical.
chemical.
physical.
physical.
Is aging primarily an example of a physical or a chemical change?
The changes that occur as we age involve the chemical reformation of our biomolecules. These are chemical changes.
How are compounds different from elements?
An element is a material in which all the atoms have the same atomic number. In other words, an element is a material made of only one kind of atom. Pure gold is an example of an element because it consists of only gold atoms. A compound is a material made of more than one kind of atom. Furthermore, these atoms typically combine in a simple whole number ratio. Water is an example of a compound because it is made of both hydrogen and oxygen atoms. The fundamental unit of water is the water molecule, which contains two hydrogen atoms for every one oxygen atom and has the formula .
Oxygen atoms are used to make water molecules. Does this mean that oxygen, O2, and water, H2O, have similar properties? Why do we drown when we breathe in water despite all the oxygen atoms present in this material?
All the oxygen water present is bound to hydrogen atoms making water molecules. Water is uniquely different from the elements oxygen, , and hydrogen, H2, from which it can be made. The oxygen our bodies are designed to breathe is gaseous molecular oxygen, . We drown when we breathe in water because it contains so little . Although they both contain oxygen, gaseous oxygen, , and water, , are vastly different materials.
Why is water not classified as an element?
Water use to be classified as an element but that was before people recognized that the basic building block of matter are these tiny particles called atoms. Today, an element is identified as a material consisting of only one kind of atom.
What is the chemical formula for the compound dihydrogen sulfide?
What is the chemical formula for the compound dihydrogen sulfide?
What is the common name for dioxygen oxide?
Ozone, .
Is nanotechnology the result of basic or applied research?
Basic research is the study of the fundamental workings of nature. As we learn about these fundamental workings, we become equipped to develop useful technological applications, which is the focus of applied research. Nanotechnology was certainly made possible because of all that we learned from basic research. Any form of technology, however, is generally the result of applied research.
People often behave differently in a group compared to when they are alone. Explain how this is similar to the behavior of atoms. Is this good news or bad news for the development of nanotechnology?
A macroscopic sample of an element contains billions upon billions of atoms bunched together. The properties exhibited by these grouped atoms are not necessarily the same as the properties of the atoms when isolated individually or in smaller groupings. Individual gold atoms, for example, appear red—not gold—and single sheets of graphite are transparent—not black. This is good news for nanotechnology because it means there are many nanoscopic properties yet to be discovered and exploited.
Know this
To name a compound IMPORTANT!!
GUIDELINE 1 The name of the element farther to the left in the periodic table is followed by the name of the element farther to the right, with the suffix -ide added to the name of the latter:
GUIDELINE 2 When two or more compounds have different numbers of the same elements, prefixes are added to remove the ambiguity. This occurs primarily with compounds of nonmetals. The first four prefixes are mono- (one), di- (two), tri- (three), and tetra- (four). The prefix mono-, however, is commonly omitted from the beginning of the first word of the name:
GUIDELINE 3 Atoms can clump together to form a molecular unit that acts as a single electrically charged group, called a polyatomic ion. For example, a carbon atom can join with three oxygen atoms to form what is known as a carbonate ion, . Some commonly encountered polyatomic ions are listed in Table 11.1. Note that most of them are negatively charged. Negatively charged polyatomic ions are placed at the end of the name. An example is lithium nitrate, LiNO3. Positively charged polyatomic ions are placed first in the name (without the word ion). An example is ammonium chloride, NH4Cl.
A polyatomic ion may appear more than once within a compound. This is indicated by placing the polyatomic ion in parentheses. A subscript just outside the parentheses indicates the number of times the polyatomic ion appears. To keep it simple, the prefixes mono-, di-, tri-, and tetra- are commonly not included for polyatomic ions:
GUIDELINE 4 Many compounds are assigned common names that are more convenient or have been used traditionally for many years. Some common names are water for H2O, ammonia for NH3, and methane for CH4.