05 Metals and Ceramics 2
1/82
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
83 Terms
What is a single crystall? (3)
Perfect periodic and repeated arrangement of atoms throughout the entire specimen
All unit cells interlock the same way and have same orientation
Naturally or artificially produced
What are polycrystalline materials?
Collection of many small crystals or grains with different crystallographic orientations, sperated by grain boundaries
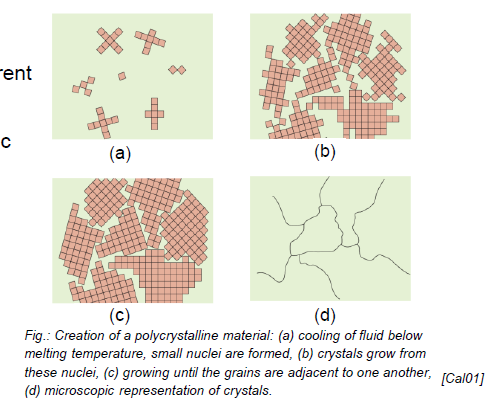
What is an isotrpoic material?
Physical properties independent of direction
Polycrystalline materials are normaly isotropic (properties average in any direction due to random orientation)
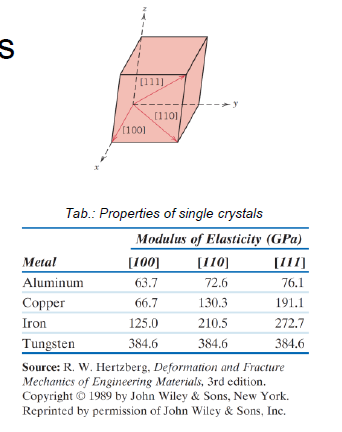
What is an anisontropic material?
Directional dependency of physical properties of single crystalls
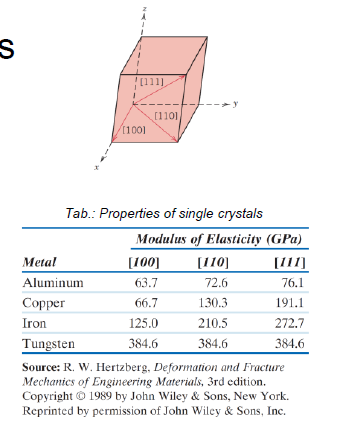
What are the characteristicas of a larger grain size? (3)
Higher melting temperatures (?)
Higher creep resistance
What are the characteristics of a smaller grain size? (2)
Better boundary sliding, thus higher creep (higher surface to slide)
Better toughness and strength
Are steels mono- or polycrystalline?
Normally polycrystalline
Single crystalline vs polycrystalline
Def: Diffusion
Material transport by atomic motion
Used to improve amterial properties
e.g. case hardened steel with carburized out case for surface hardening, fatigue and wear resistance (cf. pic, where case is harder than core region)
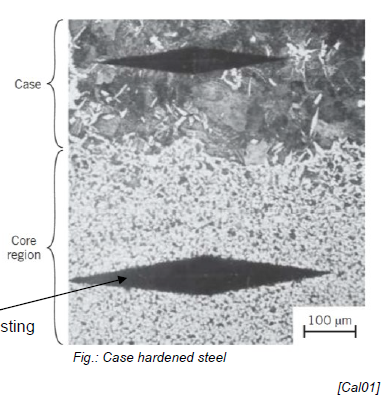
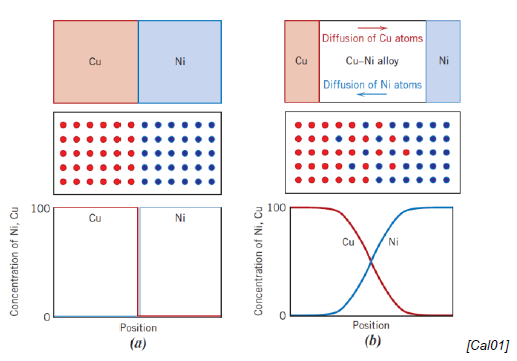
How does diffusion happen at an atomic scale?
Intimate contact between diffusion couple
Couple heated to elevated temps below melting temp
Atoms diffuse into one another
Cooling stops the process
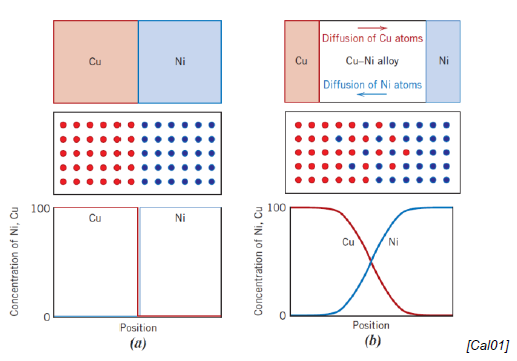
Which factor influence diffusion?
Diffusion couple
Temperature
What are phase diagrams? (2)
Graphical representation of microstructural physical states and transformations fo a material as a function of alloy constituents and temperature
For metals, binary phase diagrams (variable temperature and composition at constant pressure, usually for two components (binary allows)) are normally used
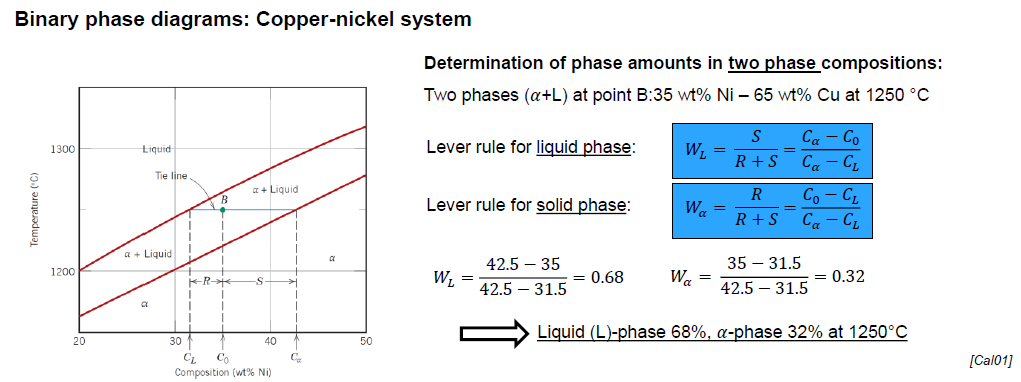
What are the regions of a Copper-Nickel diagram?
Liquid (L)
Solid (𝛼)
Two phase (𝛼 + L) lines
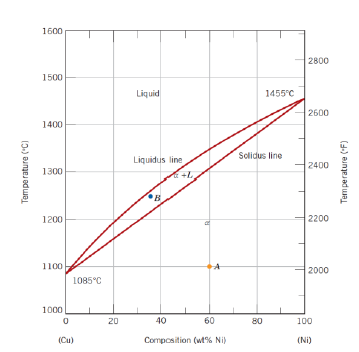
How are the regions to be interpreted?
Above the liquidus line: system is liquid
Below solidus line: system is solid
Between liquidus and solidus lines: system is in two phases
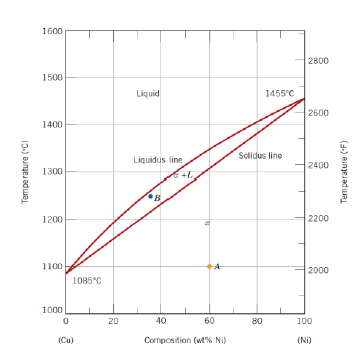
What is the meaning of the intersection points of the liquidus and solidus line?
Melting temperatures of each of the components in their pure state
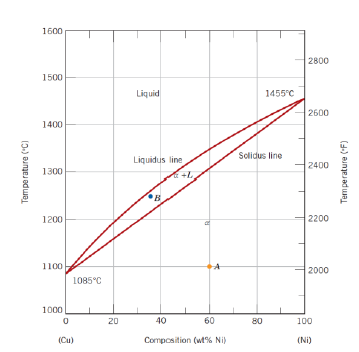
What happens when cooling down a the system from a high temperature? (3)
Solidification of the components start after crossing the liquidus line
Composition of the solid phase is not necessarily the same as the composition of the liquid phase, although the overall composition is the one designated by the vertical line
The system keeps on solidifying until crossing the solidus line, after which is a solid of composition defined by the vertical line
How can the composition of the solid phase on the biphase region be read?
Intersection of an horizontal line from the considered point to the solidus line allows to read the composition
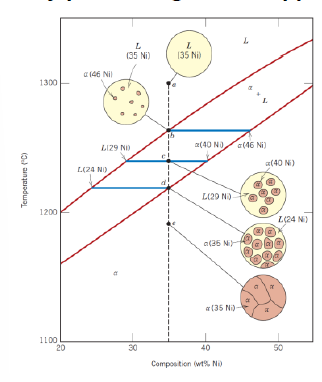
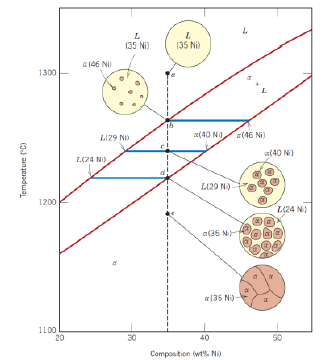
How can phase amount be determined in two phases composition?
Via lever rule for liquid phase and for solid phase and the tie line (horizontal line between liquidus line and solid line and passing through the considered point)
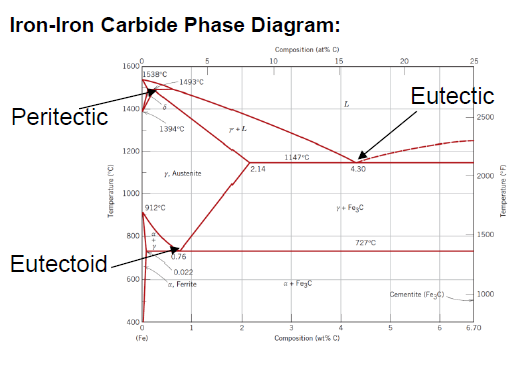
What is ferrite?
Room temperature, BCC iron
Also called α-Iron

What is austenite?
FCC iron
γ-Iron

At what temperature does pure iron ferrite experience a polymorphic transformation to austenite?
912 °C
At what temperature reverts pure iron austenite to BCC? What is the name of the new form?
1394 °C
δ-Iron (δ-ferrite)
At what temperature does pure iron melt?
1538 °C
What is cementite?
Composition of Fe and 6.70 wt% C
Fe3C
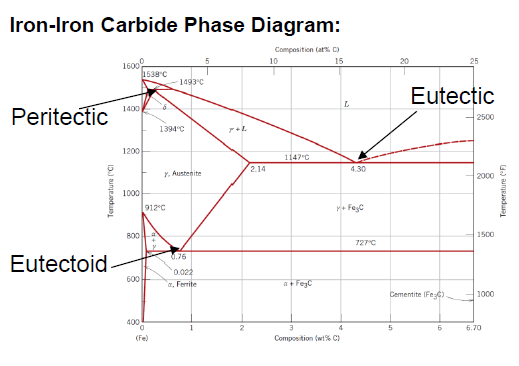
In which iron phase is carbon more soluble?
FCC γ-austenite 2.14 wt% > 0.022 wt% in α-ferrite
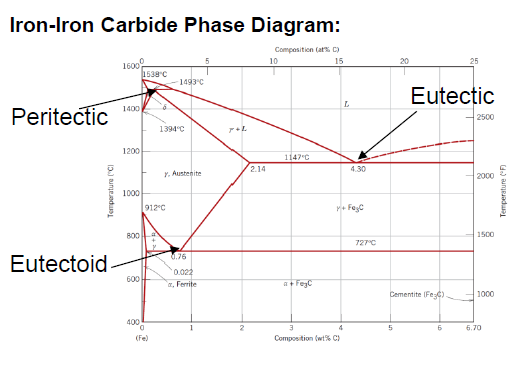
What is defined as “pure” iron?
< 0.008 wt % C
Structure made out of mostly α-Ferrite at room temperature
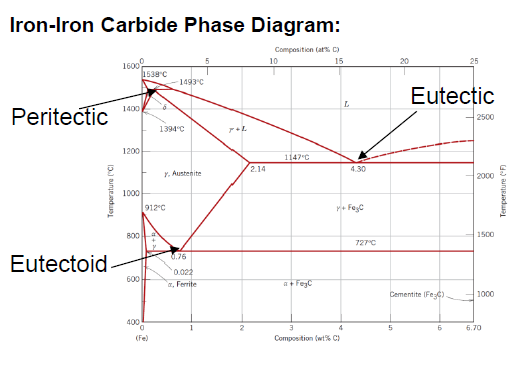
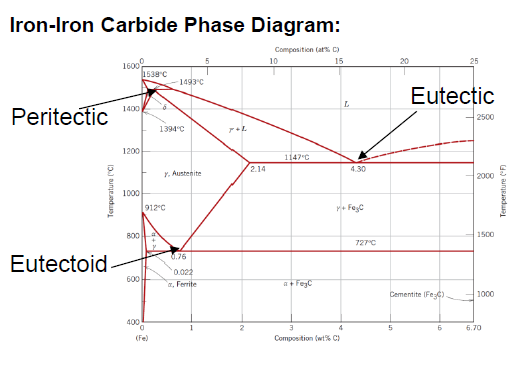
What is defined as steel?
Between 0.008 and 2.14 wt % C (typically not more than 1 wt % C)
Structure made out of mostly α-Ferrite and Fe3C at room temperature
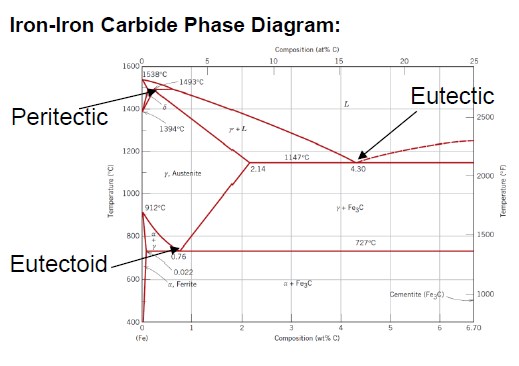
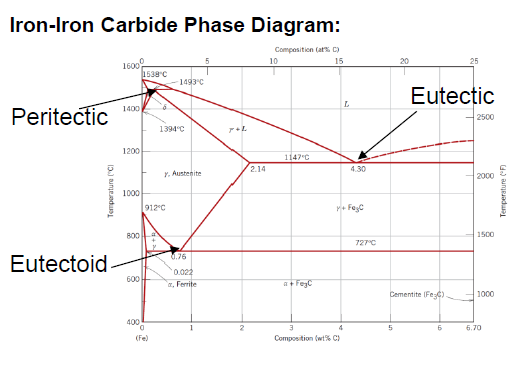
What is defined as cast iron?
Between 2.14 and 6.7 wt % C (typically less than 4.5 wt % C)
Structure made out of mostly α-Ferrite and Fe3C at room temperature
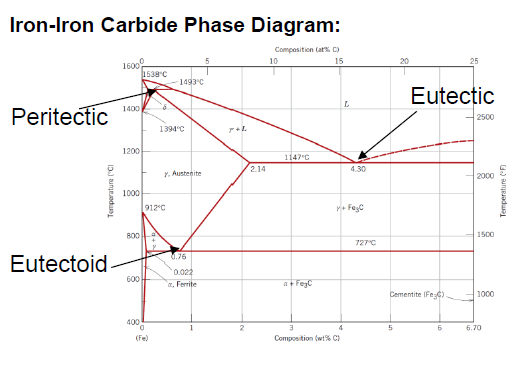
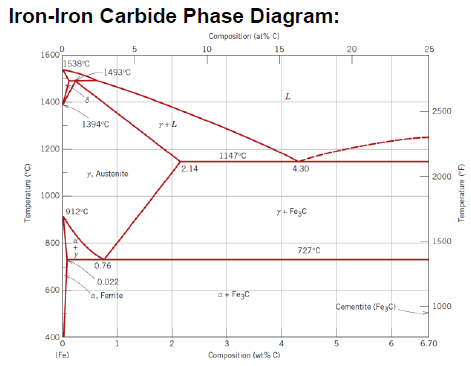
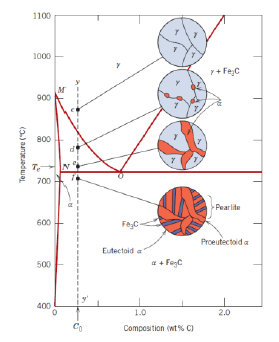
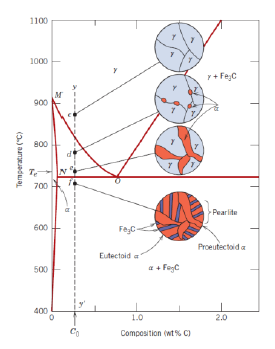
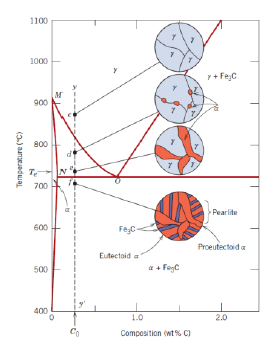
What is an eutectoid iron alloy? Where is the eutectoid point in a iron-iron carbide diagram?
A 0.76 wt % C alloy
At 0.76 wt % C at 727°C
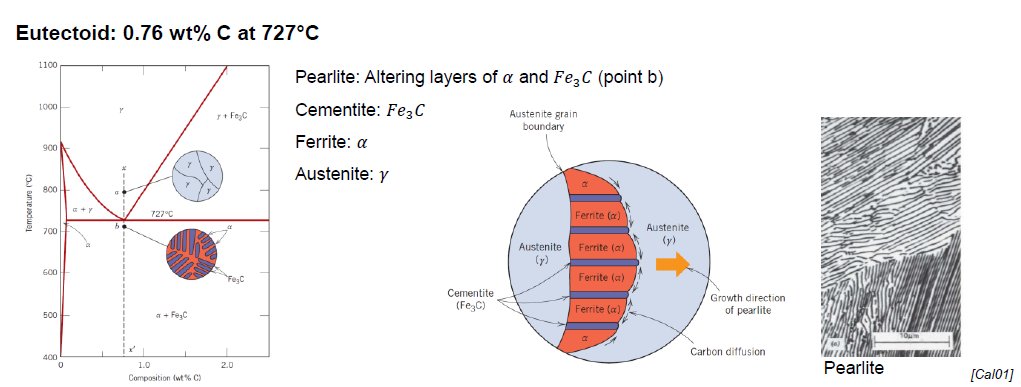
What is the eutectoid temperature?
727 °C
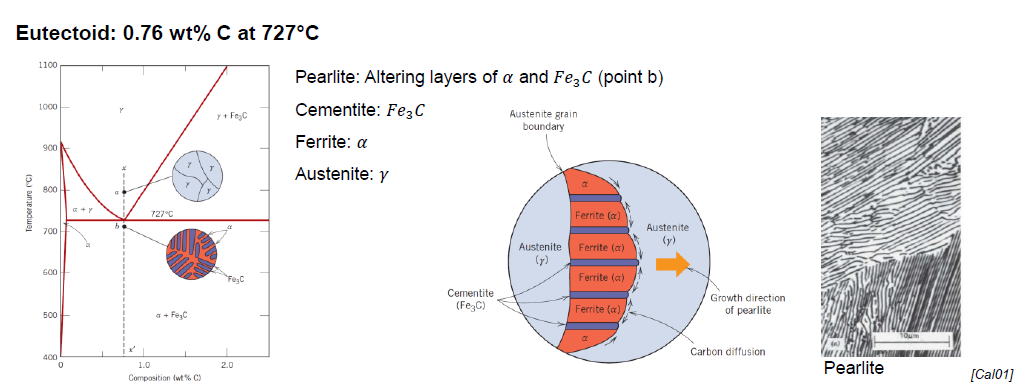
What is pearlite?
Structure made out of alternating layers of α-Ferrite and Fe3C in proportion 8 to 1
Appearance of “mother pearl“
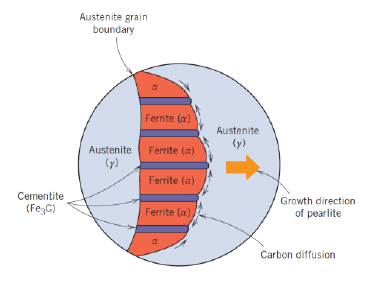
What is a hypoeutectoid alloy?
An alloy with composition ranging from 0.022 to 0.76 wt % C
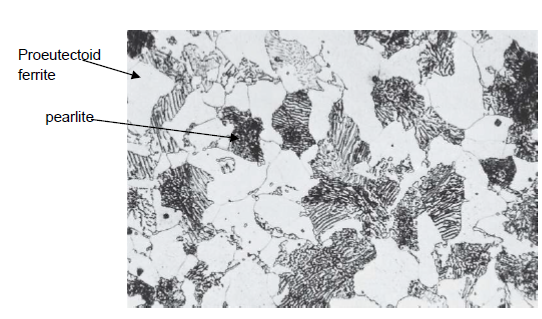
What is eutectoid ferrite?
α-Ferrite formed below a temperature sligthly above the eutectoid temperature (727 °C) present in the pearlite
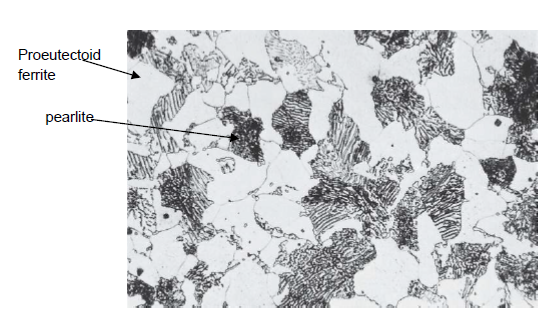
What is proeutectoid ferrite?
α-Ferrite formed above low a temperature sligthly above the eutectoid temperature (727 °C) present in the pearlite (for a hypoeutectoid alloy)
What is a hypereutectoid alloy?
An alloy with composition ranging from 0.76 to 2.14 wt % C
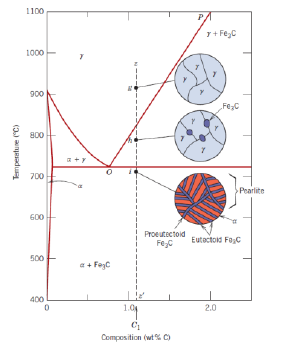
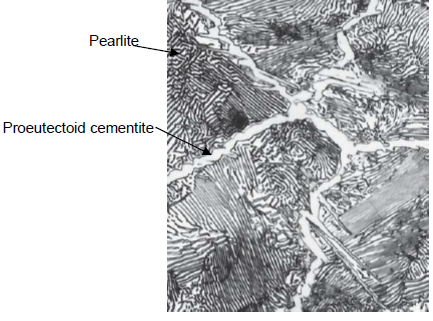
What is proeutectoid cementite?
Cementite formed above the eutectoid line (for a hypereutectoid alloy)
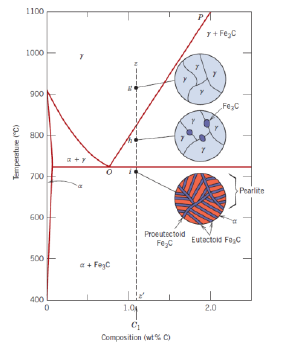
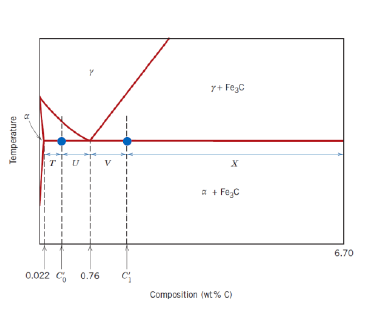
Can the relative fractions of pearlite and ferrite in hypoeutectoid alloys and of pearlite and cementite in hypereutectoid alloys be calculated?
Yes, with the respective lever rules
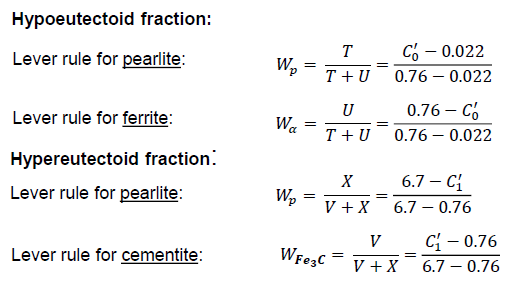
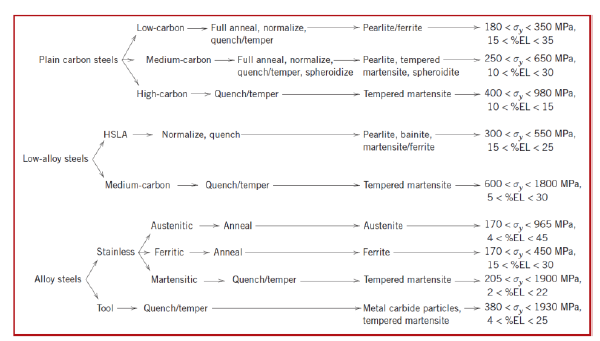
What are the classes of steel? (3)
Plain carbon steel
Low-alloy steel
Alloy steel
Def: heat treatment
Process of heating and cooling metals to change their microstructure and make their physical and mechanical properties more desirable (strength, hardness, toughness, ductility)
Depends on the temperature the metals are heated to and the rate of cooling
What are the steps of a heat treatment?
Heat
Hold
Cool
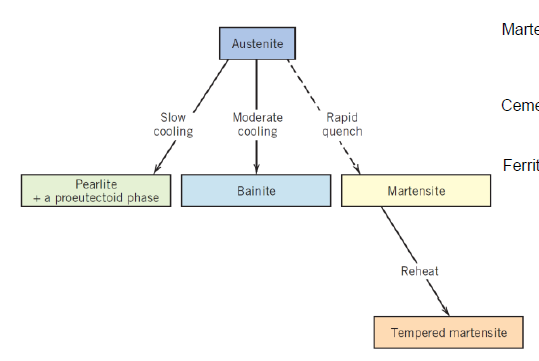
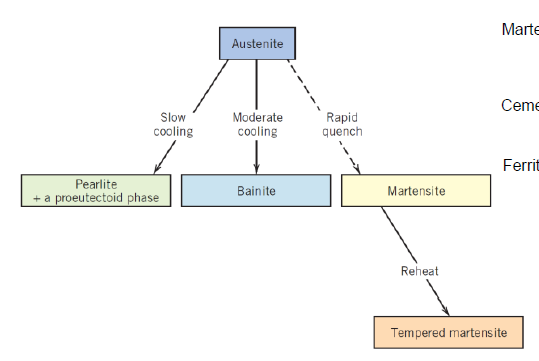
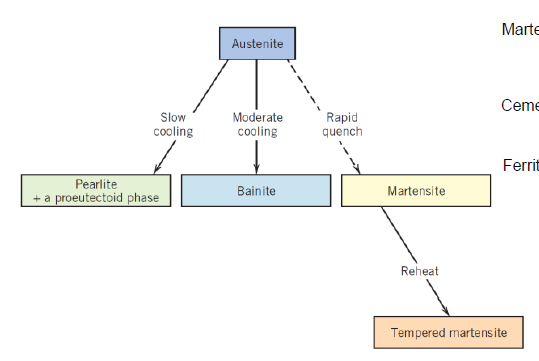
What are the main types of heat treatment? (2)
Hardening
Annealing
What is done in hardening?
Material is cooled very fast up to quenching to achieve non-equilibrium microstructure
On what depends the hardening process of a material? (3)
Alloy (composition)
Type of quenching medium
Size and shape of specimen
What is done in annealing?
Material is cooled down in slow rates after being heated up to elevated temperatures for fully diffused equilibrium mantaining microstructure
Why is annealing carried out for? (3)
Relieve stressses
Increase softness, ductility and toughness
Achieve an specific microstructure
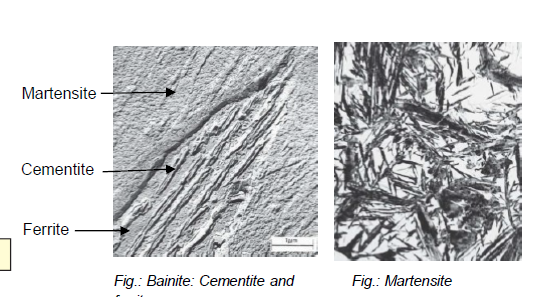
What is bainite?
Fe-Fe3C composition (analogous to pearlite) formed by cooling austenite moderately
In form of needles/plates
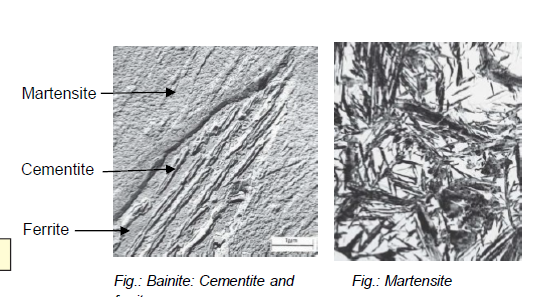
What is martensite?
Fe-Fe3C composition (analogous to pearlite) formed by cooling austenite by rapid quenching (hardening)
Non-equilibrium single-phase
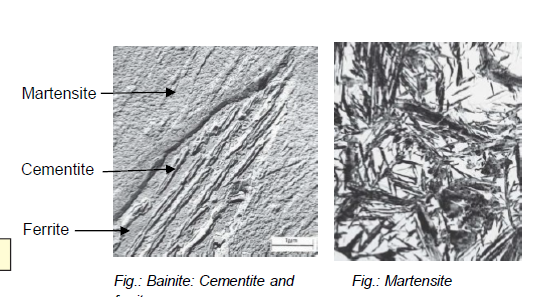
On what does it depend to get ferrite, cementite or martensite?
Ferrite: low carbon, slow cooling
Cementite: high carbon, slow cooling
Martensite: rapid quenching
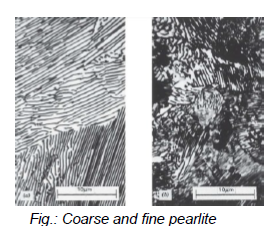
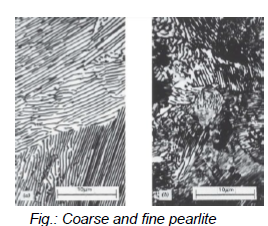
What is the difference between coarse and fine pearlite?
Coarse: just above eutectoid temperature → thick lamellae
Fine perlite: below eutectoid temperature (540°C) → thin lamellae
Both are two-phase compositions
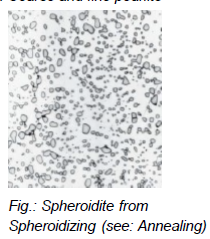
What is spheroidite?
Pearlite/bainite left for long time below eutectoid temperature
Fe3C embedded in a α-ferrite matrix
What is normalizing? (within annealing)
Removal of uneven structures from the microstructure for uniform grain with optimum strength and toughness
Fast heating, short holding times, fast cooling (not quenching)
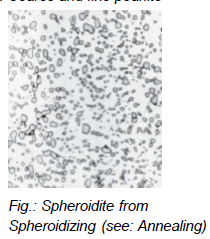
What is spheroidizing? (within annealing)
Spheroidized steels (soft and ductile) for machining
Annealing under eutectoid temperature
What is stress relief annealing?
Relief stress from previous process and heat tretments
Temperature below property change and higher than service
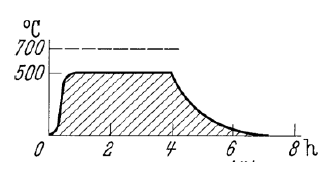
What is homogenizing? (within annealing)
Homogenizig local differences in microstucture for heterogenous grain distribution
High temperatures and long times
What is coarse grain annealing? (within annealing)
Formation of large grain structures to improve machinability
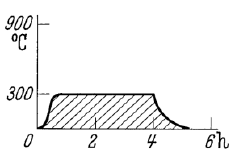
What is tempering? (within annealing)
Low temperatures, short time to make martensite more ductile and relieve stresses
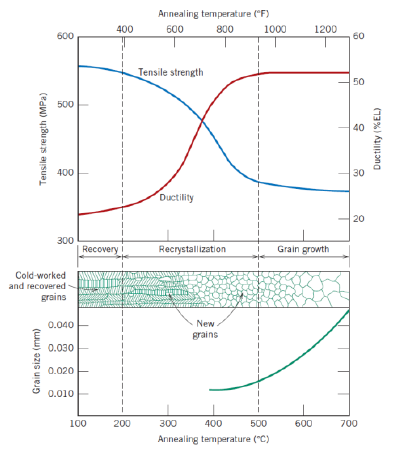
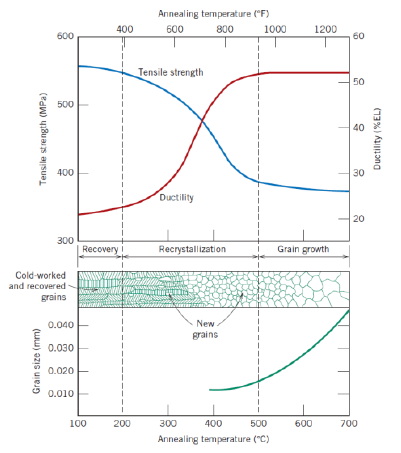
Def: recovery (2)
Process in which some stored internal strain energy is relieved in which a reduction of the number od dislocations takes place
Physical properties are recovered
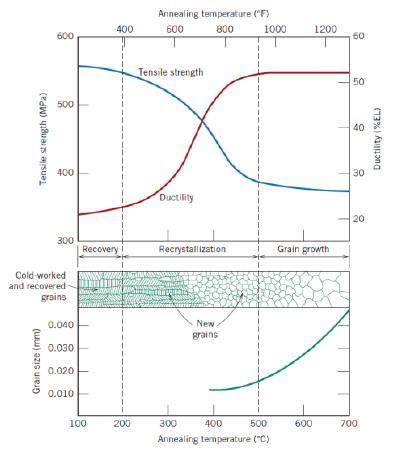
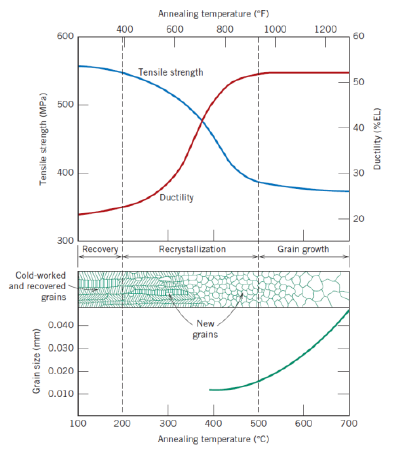
Def: recrystallization (2)
Fromation of new, strain-free and equiaxed grains
Metal becomes softer and weaker, and more ductile
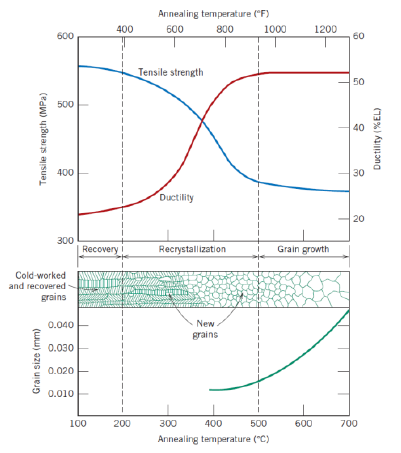
Def: recrystallization temperature
Temperature at which recrystallization is completed in 1 h
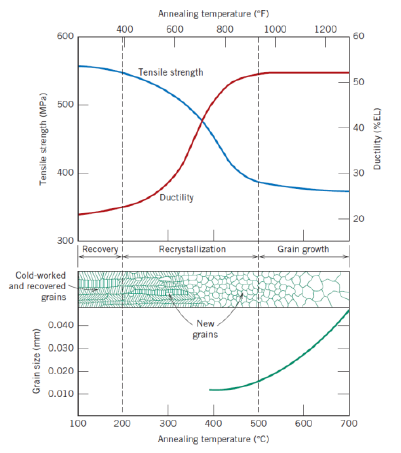
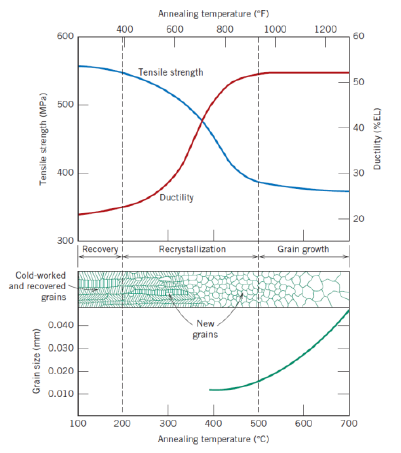
Def: grain growth (2)
Decrease of total boundary area
Increase of average grain size
Further reduction of tensile stress
Whar are ferrous alloys?
Allows in which iron is the prime constituent
What are steels?
Iron alloys within 0.008 and 2.14 wt % C
What are Low-Carbon Steels? (4)
Less than about 0.25 wt% C
No martensite formation from heat treatments
Soft, weak, ductile and tough alloys
Cheap
What is HSLA? (4)
High strength, low alloy
Steel with other allowing elements under 10 wt %
Higher strengths after heat treatments
Ductile, formable, machineable
What are medium carbon steels? (4)
Steel ranging between 0.25 and 0.6 wt % C
Heat treated to improve mechanical properties
Stronger than low.carbon steels, lower ductility and toughness
High.strength and toughness applications
What are high-carbon steels? (3)
BEtween 0.60 and 1.4 wt % C
Hardest and strongest
Less ductile
Tool steel
What are stainless steels? (6)
Corrosion resistant
At least 11 wt % Cr
Corrosion enhancement with Ni and Mo
Martensitic, ferritic or austenitic (the latter two not heat treatable but cold workable)
Marteniste and ferrite magnetic, austenite not
High temperature resistance
SQ: What are single crystals?
Perfect periodic and repeated arrangement of atoms throughout specimen
All unit cells interlocked in the same way
SQ: What are polycrystalline materials?
Comprised of numerous small crystals or grains with different crystallographic orientations
Grain boundaries between grains
SQ: What are the influencing parameters of diffusion processes?
Material combination (diffusion couple)
Temperature
Intimate contact
SQ: What are phase diagrams used for?
To visualize the behavior of an alloy exposed to different temperatures as a function of its relative composition
SQ: Where is the peritectic, eutectic and eutectoid point in the iron carbide diagram?
Peritectic: (0.18 wt % C; 1493 °C)
Eutectic: (4.30 wt % C; 1147 °C)
Eutectoid: (0.76 wt % C; 726 °C)
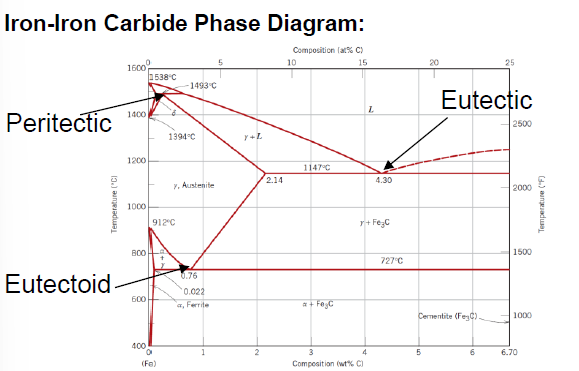
SQ: Which carbon contents define iron, steel and cast iron?
Iron: 0 - 0.008 wt % C
Steel: 0.008 - 2.14 wt % C
Cast Iron: 2.14 - 6.7 wt % C
SQ: How can the phase fractions and total structure be calculated?
With the respective lever rules
SQ: Draw and name the microstructures of steel into the iron-iron carbide diagram?
γ, α + Fe3C (eutectoid)
γ, α + γ, α + Fe3C (hypoeutectoid)
γ, γ + Fe3C, α + Fe3C (hypereutectoid)
SQ: Why is heat treatment performed on steels?
To modify microstructure
To achieve wished toughness, hardness, ductility, strength
Relieve internal stress
SQ: Fill out the annealing areas in the iron carbide diagram
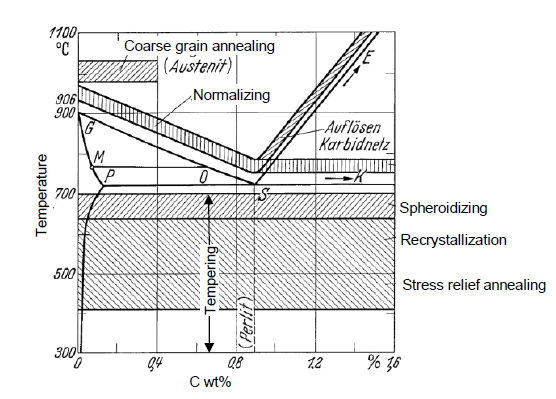
SQ: What is the difference between annealing and hardening?
Hardening: drastic heating, rapid cooling (quenching), non-equilibrium microstructure (martensite)
Annealing: heating, hold, slwoly cool down, microstructure fully diffused and in equilibrium, higher ductility and toughness
SQ: Name 5 typical alloy elements of steel
Chromium
Vanadium
Molybdenium
Nickel
Titanium
Copper
Manganese
Carbon
SQ: What C contents have low, mid and high carbon steels?
Low Carbon Steel: 0.008 - 0.25 wt % C
Medium Carbon Steel: 0.25 - 0.60 wt % C
High Carbon Steel: 0.60 - 1.4 wt % C
SQ: Why is not always high-carbon steel used?
Lower ductility and toughness
Difficult machinability and weldability
SQ: Why is the main advantage of stainless steel?
Corrosion resistant
Low creep rates under temperature
SQ: Why is stainless steel corrosion resistant?
High content of Chromium (more than 11 wt %)