chapter 5 enantiomers and diasteromers
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
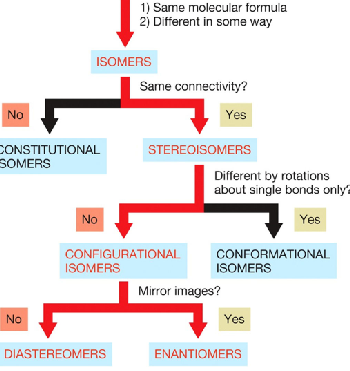
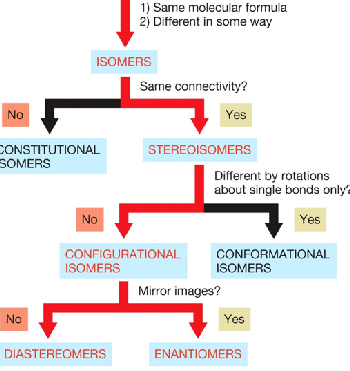
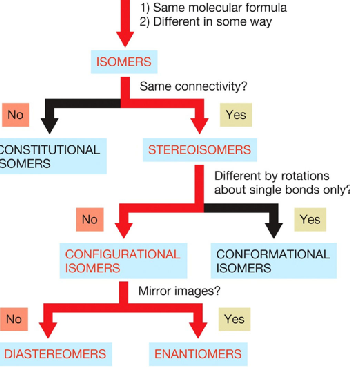
configurational isomers
have the same connectivity, but differ in a way other than by rotations about single bonds and includes two types
enantiomers
diasteromers
enantiomers
configuational isomers that are nonidentical or nonsuperimposable mirror images
diastereomers
configurational isomers that are not mirror images of each other. Also nonsuperimposable
what does it mean for a molecule to be chiral
it has “handedness, and can exist as two enatiomers. often has a carbon with 4 different groups attached
what does achrial mean?
a molecule that is not chrial; its mirror image is not super imposable ( means that one object can be placed on top of another so that their corresponding parts completely overlap and align, making them effectively identical)
how can you tell if a molecule is chiral
look for a carbon with four different groups attached. if it has one, its chiral
give a real world analogy for enatiomers
left and right hands: mirror images but cannot perfectly overlap
stereocenter
an atom with the property that interchanging any two of its attached groups produces a different stereoisomer
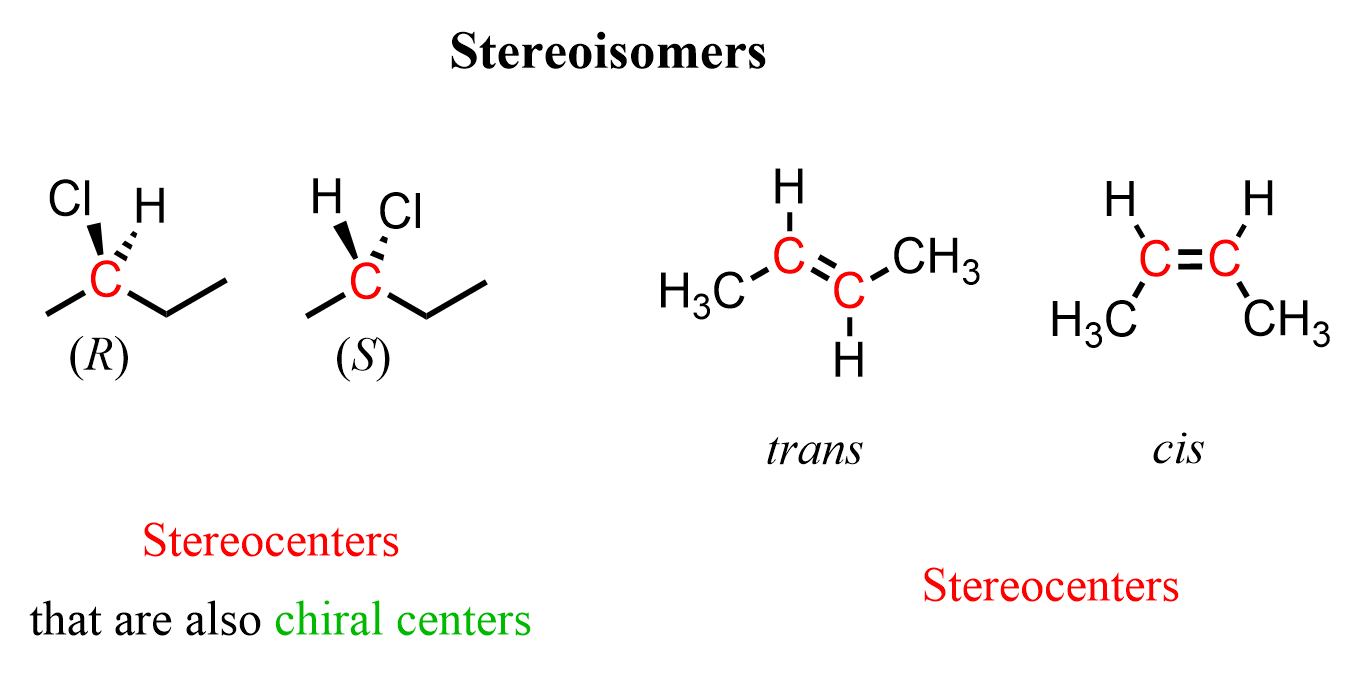
what is a tetrahedral stereocenter
a chiral center that has 4 different groups attached to it
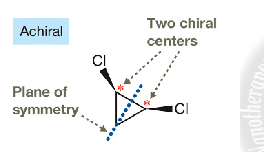
meso compounds
a molecule is meso if it contains at least two chiral centers but has a plane of symmetry that makes it achiral overall.
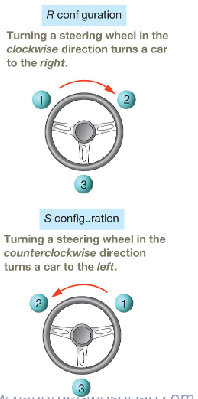
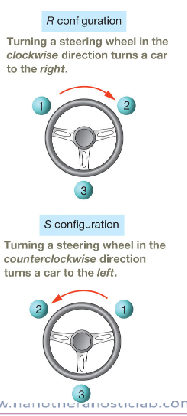
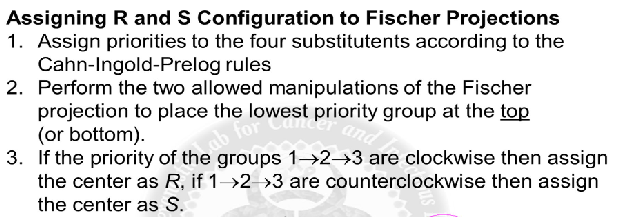
IUPAC rules for assigning configuration at the chiral center
Assign priorities, 1 through 4, to the substituents based on
atom directly connected to chiral C. The highest atomic
number is highest priority,1, and the lowest priority is 4.
2. Orient the molecule properly so the lowest-priority substituent
points away.
3. Observe the arrangement of substituents 1 through 3 and
assign the configuration as R or S.
a. If the substituents having priorities 1 through 3 are
arranged clockwise, then the chiral center is R
configuration.
b. If the substituents are arranged counterclockwise, then the
chiral center is S configuration.
number 4/hydrogen has to be pointed towards the back, which may require flipping the molecule /way of rotation
how to determine the maximum number of steroisomers
Maximum number of stereoisomers = 2 n.
where n = number of chiral centers/structural units capable

describe r vs s configuration
see image for configurations
diasteromers are:
hint 4 characteristics:
steriorismers that are non enatiomers
steriosomers that are not mirror images
geometric isomers (cis trans)
molecules with 2 or more chiral carbons
fischer projections
convient way to depict complex molecules with more than one sterocenter/chiral carbon
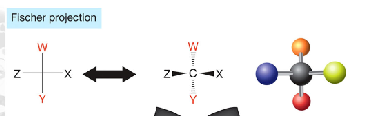
what is a characteristic of fischer projections, think rotations
you can only rotate them 180 degrees unless you are holding one atom constant because a 90 degree rotation inversts the stereochemistry and is illegal
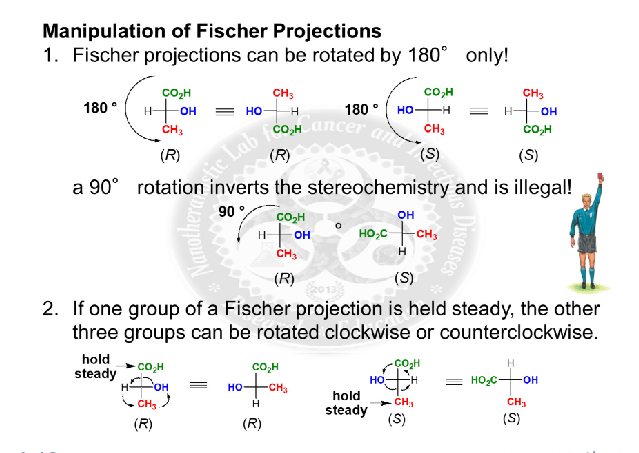
Assigning R and S configuration to fischer projections
see image for fischer project configurations assigning
explain how bonds point in fischer projections
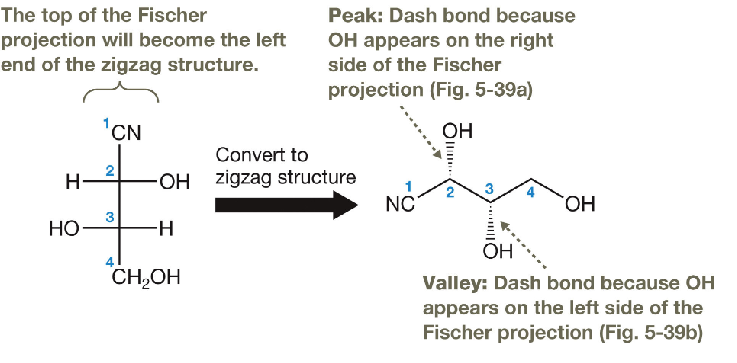
vertical bonds in a fischer projection point away from you and horizontal bonds point toward you.
converting a Fischer projection ot a zigzag conformation
see image for the conversion
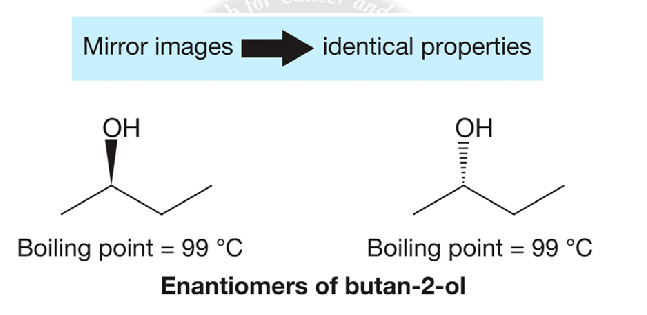
Enatiomers
mirror images that have the same connectivity and preciesly the same polarity
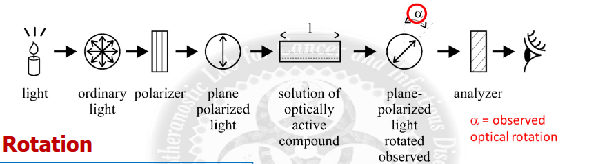
how is a polarimeter used?
a device for measuring the optical activity of a chiral compound / degree of rotation
enatiomeric excess (aka optical purity) formula
can also be calculated from optical rotations
% enantiomeric excess = ( observed specific rotation / specific rotaion of the pure enatiomers) *100

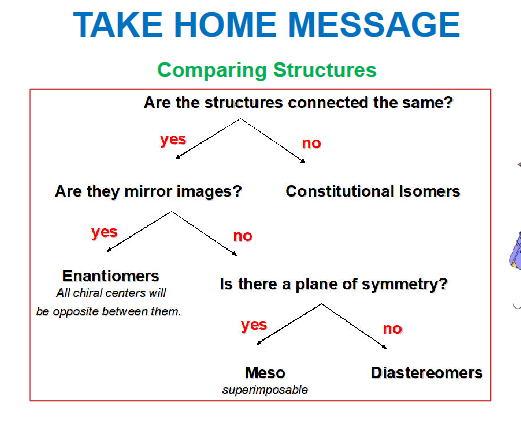
take home message of comparing structure
see image on comparing structures