CH13 Alkenes
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
What types of bonds are in alkenes?
Sigma and pi
How is a pi bond formed and what does it do?
By the sideways overlap of 2 p-orbitals - one from each carbon atom of the double bond.
The bond locks the 2 carbon atoms in position and prevents them from rotating.
What is a steroisomer?
Have the same structural formula but a different arrangement of atoms in space
What is cis-trans isomerism?
Molecules must have a c=c bond and each carbon atom must be attached to 2 different groups but one of the groups must be hydrogen
What is e/z isomerism?
A molecule that has a c=c bond and different groups attached to each carbon atom of the double bond.
Equation for hydrogenation of alkenes and the conditions required and the reaction name
C3H6 + H2 > C3H8
Requires a nickel catalyst
Addition reaction
Equation for halogenating of alkenes (with a halogen) with conditions and name of reaction
C3H6 + Br2 > C3H6Br2
Room temperature
Addition reaction
Equation for hydration of alkenes with conditions and name of reaction
C3H6 + H2O > propan-1-ol’ OR propan-2-ol’
H3PO4 catalyst
Addition reaction
What is an electrophile?
An atom or group of atoms that is attracted to an electron-rich centre and depts an electron pair.
Equation for reaction between butane and hydrogen bromide/bromine and name of reaction.
C4H8 + HBr > C4H9BR
Electrophillic addition
Attempt to draw out the mechanism for the reaction between butene and hydrogen bromide
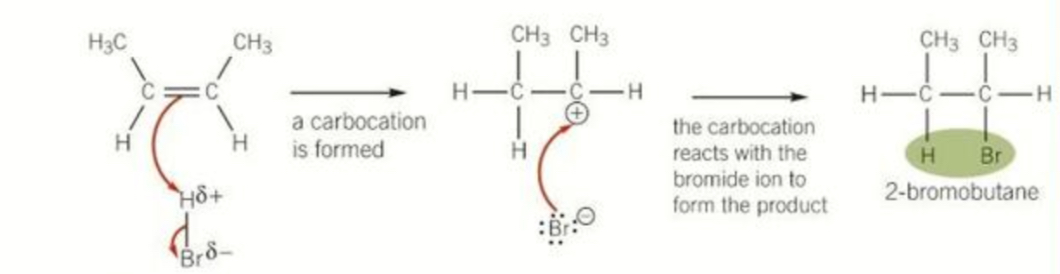
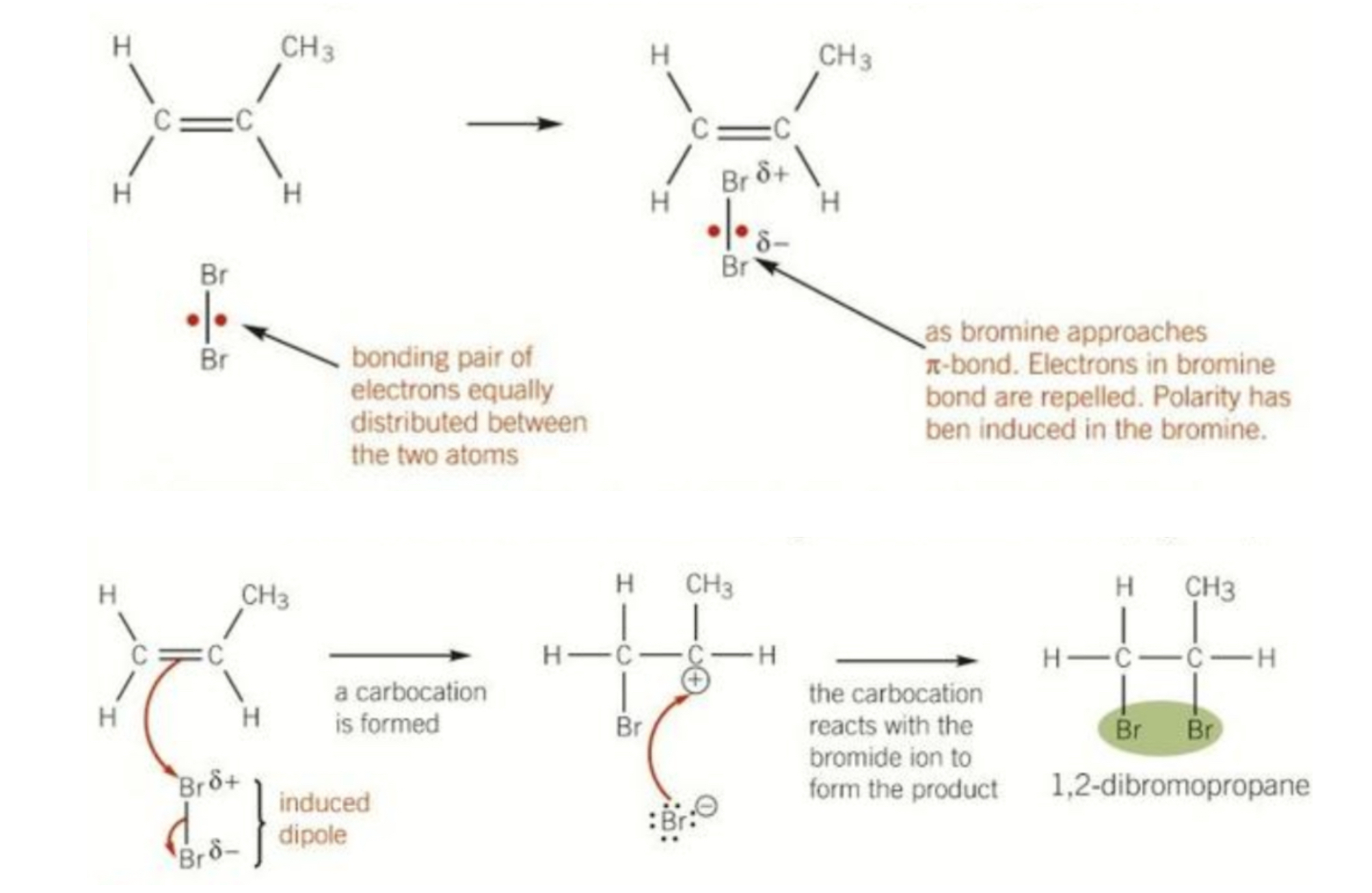
Mechanism for reaction of propane with bromine drawing
What is addition polymerisation?
Alkene molecules undergoing a reaction to produce long saturated chains containing no double bonds.
What is poly(ethene) used in?
Plastic bags, shampoo bottles, kids toys
Pros and cons of polymers for the environment
Pros: lack reactivity, can be recycled to reduce use of finite resources, PVC cannot be dumped as when burned it releases hydrogen chloride which is dangerous.
Cons: non-biodegradable (can kill marine life), some are difficult to recycle