wagner chem lesson 2
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
Aufbau Principle
An electron occupies the lowest-energy orbital one at a time
atomic theory explains?
how chemical reactions occur
exited state
electrons jump to high energy orbitals
The center of an atom contains most of the mass and is positively charged the electrons move in the case empty space around the nucleus
Rutherford's model
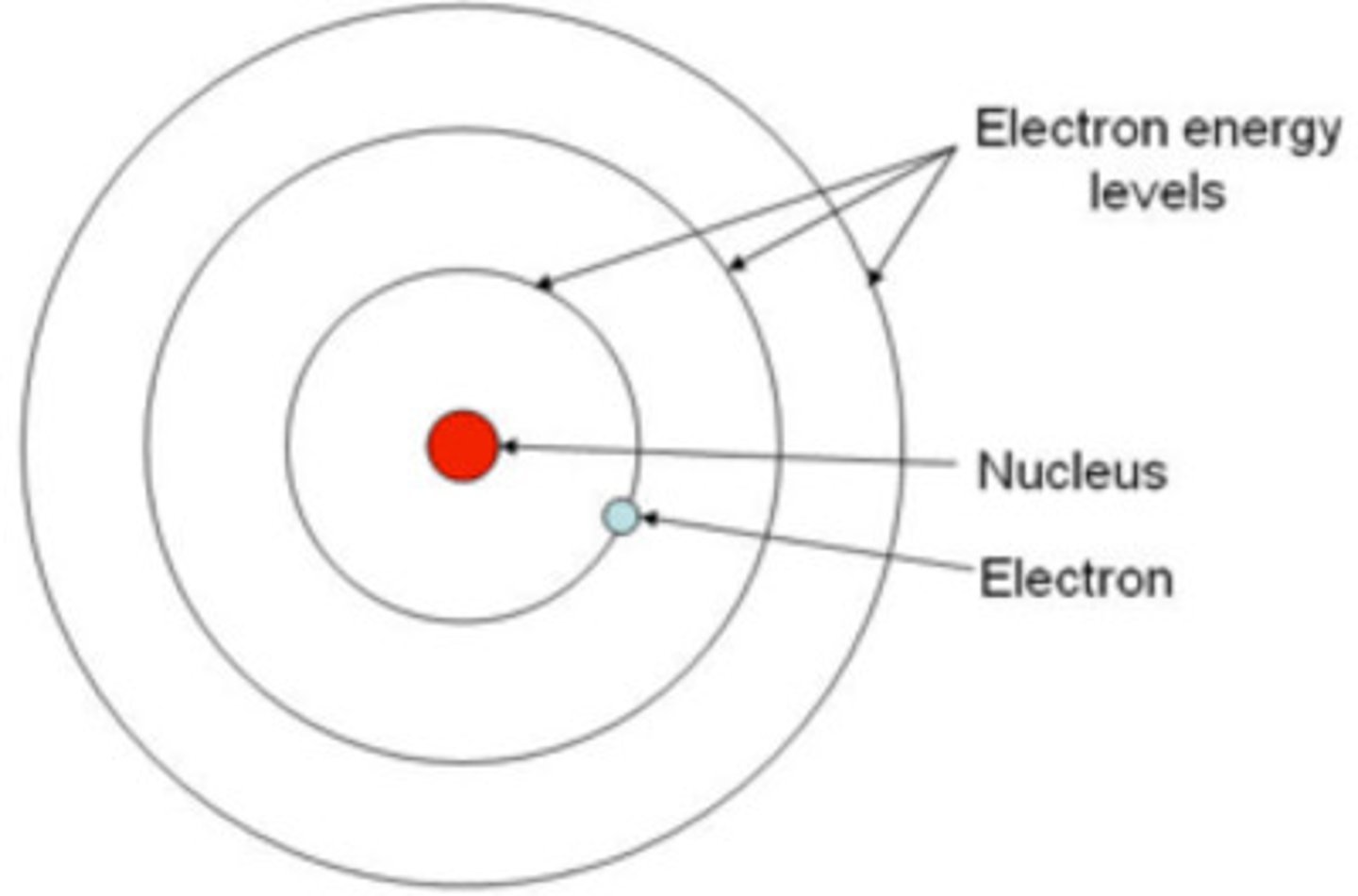
electrons have orbits around the nucleolus each orbit has a cerein energy level.
the bohr model
neutral atoms
same number of neutrons and protons
same number of protons and neutrons
isotopes
cations and anions
cat=positive an=negative
Pauli Principle
orbitals can hold two electrons with opposite spins
hund's rule
electrons don't pair until each of the lowest energy orbitals are full
ground state
electrons jump down to lower energy orbitals
Greek philosopher with an idea, no experimental proof.
Democritus
Atoms are little solid spheres
Daltons model
positive and negative charges inside the atom
Thompsons model
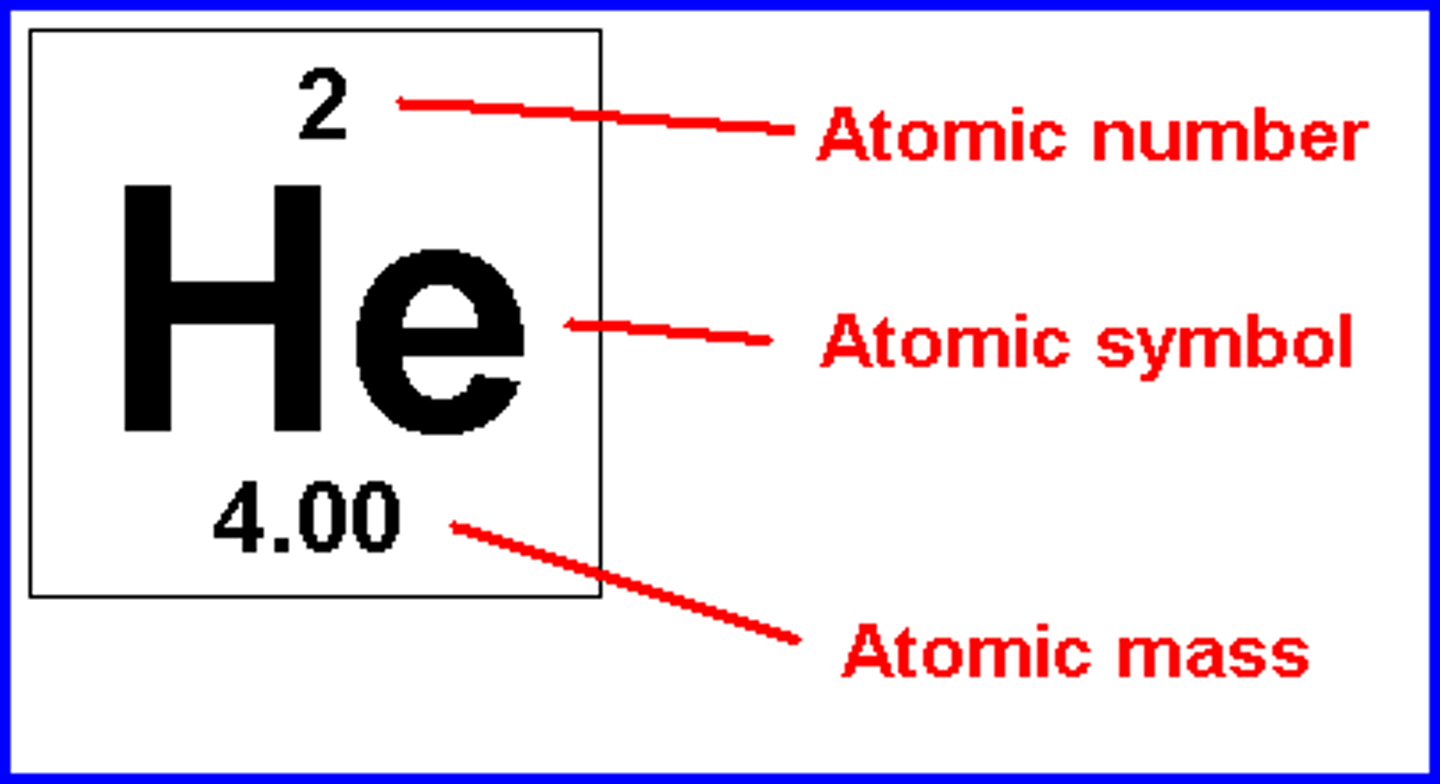
number at top
atomic number (number of protons)
ions
charged atoms