Chapter 7 - Spectroscopy
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
Rotational spectroscopy
Very little energy is needed to change the rotational state of a molecule and the electromagnetic radiation absorbed or emitted lies in the microwave region.
Rotational spectroscopy is therefore also known as microwave spectroscopy. Pure rotation spectra are discrete line spectra.
Vibrational spectroscopy
All molecules are capable of vibrating.
For complicated molecules the number of vibration modes is very large
They typical distance between vibration levels is ont he order of 10-20 to 10-19 J, which corresponds to frequencies in the infrared region of the electromagnetic spectrum.
Therefore vibration spectroscopy is also called infrared spectroscopy.
Electronic transitions
The energy needed to change the occupation of the orbitals in a molecules is on the order of 10-19 to 10-18 J
Consequently, the photons absorbed or emitted when such transitions occur lie in the ultraviolet (UV) visible region of the electromagnetic spectrum.
The technique to probe this is called UV/Vis spectroscopy
These transitions are always accompanied by a wide range of transitions in vibrational and rotational states. Therefore the spectrum consists of many lines.
Fluorescence
Some molecules discard their excess energy by emitting radiation.
When an electron relaxes back from the excited state to the ground state, it emits a photon. This process is called fluorescence.
When a photon is absorbed, it takes the molecule into an excited electronic state
Production of a photon
Photon is absorbed, part of the energy is quickly given to the surroundings.
The molecule gives up vibrational energy as it collides with other molecules.
However the surrounding molecules might be unable to accept the larger energy needed to lower the molecule to the ground electronic state.
In this case the molecule can generate a photon and emits this as radiation as it falls back to the ground state.
This photon is fluorescent light.
Lambert-Beer law
E is the molar absorption coefficient.
C is expressed in mol/L (concentration)
and b in cm (width of cuvette)
So the dimension of e is L mol-1 cm-1
I is the light intensity, and I0 is the initial light intensity before its shined through the sample.
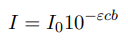
Lambert-Beer’s law for mixtures
Where ei and ci are the extinction coefficient and the concentration of species i.
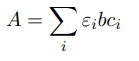
What is transmittance?
Fraction (or %) of photons not absorbed
Calculated by I/I0
What is the fingerprint region
The vibration of the whole molecule
What is phosphorescence
Same as fluorescence but slower.