Atomic structure
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
Why is the mass number (top number) a decimal?
Because of the presence of isotopes
Define isotopes
Atoms of the same element with different numbers of neutrons and masses
Why do all isotopes of an element react in the same way?
They have the same electron configuration
What does abundance tell you?
How common each isotope is
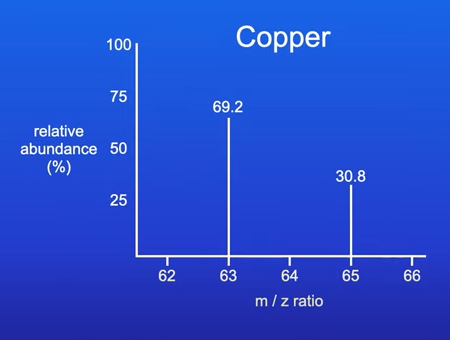
E.g. 69% of copper atoms are copper 63
31% of copper atoms are copper 65
How can we determine the mass number and abundance of isotopes ?
Use a machine called a mass spectrometer
Outline the stages of the time of flight mass spectrometer
Take a sample of the element your interested in. Place it in the sample chamber
The sample contains all of the different isotopes of that element
Atoms go through a process of ionisation. Converting all the atoms into positive ions
These positive ions are now attracted to a negatively charged plate
The negatively charged plate causes the ions to accelerate increasing kinetic energy of the ions
All of the ions with the same charge will have the same kinetic energy
Once the ions pass through the negative plate. They stop accelerating
They then drift down the flight tube at different velocities .With lighter ions moving faster than heavier ions
After reaching the detector, each positive ion gains an electron from the detector.( An ion with a single positive charge will gain a single electron)
This transfer of electrons causes a current to flow
Ions of the lighter isotope will have a greater velocity and reach the detector first
Size of current produced when each isotope hits the detector is proportional to its abundance
Why is the interior of the mass spectrometer a vacuum?
To prevent the ions from colliding with air molecules
As this can cause the ions to be deflected and slowed down
Explain electron impact
Its a method of ionisation
A vapourised sample is injected at a low pressure
Electron gun fires high energy electrons at the sample
Knocking out outer electron
E.g. Ti → Ti+ + e-
Explain electrospray ionisation
Sample is dissolved in a volatile solvent
Injected through a needle connected to a high voltage
Molecules gain H+
E.g.: Mg + H+ →MgH+
Interpretating the mass spectrum
2 peaks show that Copper has 2 main isotopes with an abundance of 69.2% and 30.8%
M/Z = Ratio of the mass of each ion to its charge
All of the ions have a single positive charge so the M/Z ratio is basically the relative mass of the ion
What happens when mass spectrometry is carried out on a molecule?
A range of peaks are formed due to fragmentation
What does fragmentation do?
When fragmentation happens a bond breaks, forming a molecular ion and a radical.
What can the fragmentations be used for?
They can help identify the molecule
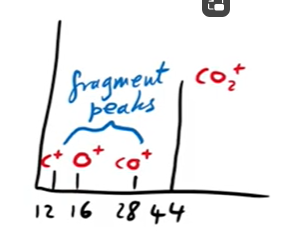
Mass spectrum for carbon dioxide
The peak at 44 shows you a molecule that has an mr of 44. It can be assumed that it is co2. This peak is called a molecular ion M+
The rest of the peaks depict other parts of the molecule that have been ionised
The other peaks give you the mr of the atoms in the molecule that help figure out what that the molecule is
The peak at 12 (mr of carbon) tells you there is a carbon in the molecule
How can the maximum number of electrons an energy level can hold be calculated
2n2
n being the energy level
E.g. the first energy level can hold 2 electrons 2(1)2
Electrons in energy levels are found in regions called……
Atomic orbitals
Define atomic orbital
A region around the nucleus that can hold up to 2 electrons with opposite spins
Shape of the s orbital
spherical
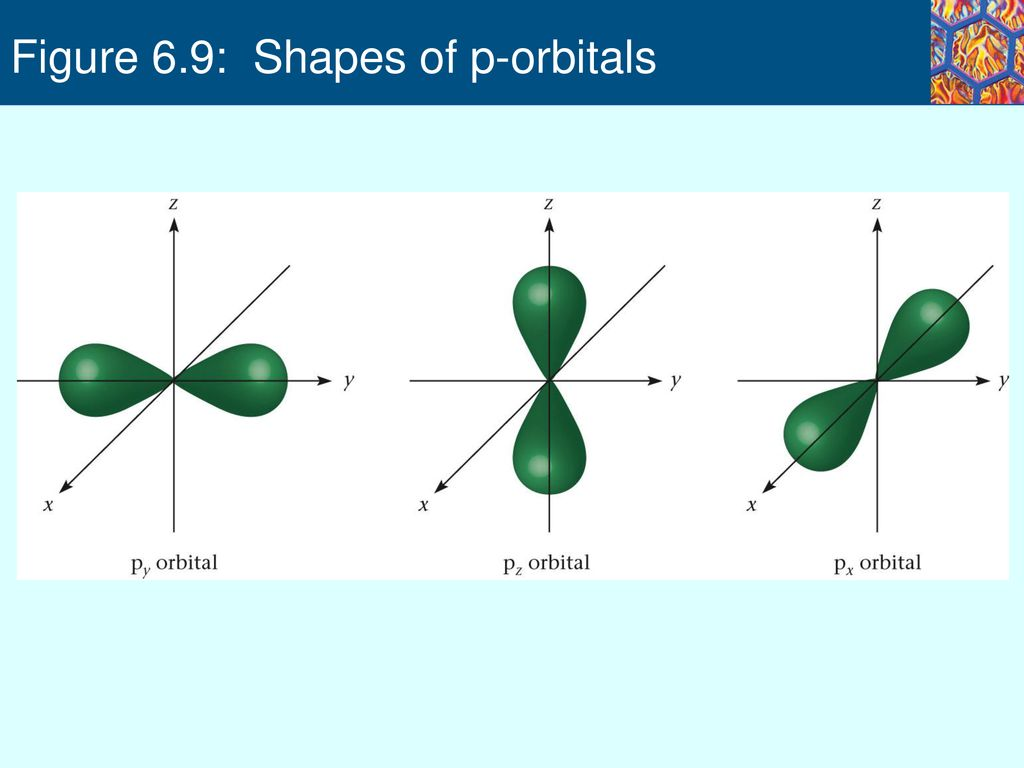
Shapes of p orbitals
What happens to the energy of the shells as you move further from the nucleus ?
energy of the shell increases
weaker electrostatic force of attraction between nucleus and electrons means electrons on shells further from the nucleus require more energy to stay in these positions
Order of orbital filling
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
Rules for filling atomic orbitals
Orbitals with the lowest energy are filled first
Can have 2 electrons in each orbital but they must have opposite spins
Fill 4s before 3d
Fill orbitals with the same energy singly, before pairing. Because electrons in the same orbital repel
Name 2 elements exempt from orbital filling rules and why
Chromium and copper
When filling the orbitals for these 2 elements put only 1 electron in 4s and the rest in 3d
Because 3d subshell is most stable when its completely or half full
Electron configuration is always written in order of …..
shells not filling
Whats the shorthand configuration for sodium

What is the shorthand configuration for sulphur?
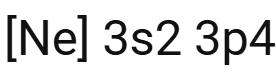
When an atom forms an ion which subshell does it loose or gain electrons from or in
The subshell with the highest energy
What is the electron configuration of an Mg atom and an Mg2+ ion?
Mg atom=1s2 2s2 2p6 3s2
Mg2+ ion = 1s2 2s2 2p6
Mg loses 2 electrons from the 3s subshell to form a positive ion
What happens when a D block element becomes an ion and why
It loses electrons from 4s subshell before 3d
The 4s subshell always filled before 3d. As 4s has lower energy than 3d
Once the 4s subshell contains electrons it has a higher energy than 3d subshell
Electron configuration for Fe and Fe2+
Fe = 1s2 2s2 2p6 3s2 3p6 3d6 4s2
Fe2+ = 1s2 2s2 2p6 3s2 3p6 3d6
Fe is a d block element
What do you need to remove an electron from an atom?
Energy
Define first ionisation energy
Energy needed to remove one mole of electrons from one mole of atoms in their gaseous state to form one mole of 1+ ions in their gaseous state
First ionisation energy equation for Mg

What is meant by successive ionisation energies?
Once you have removed 1 electron you can continue to remove electrons and measure the ionisation energy each time
Define second ionisation energy
Energy required to remove one mole of electrons from one mole of 1+ ions in their gaseous state to form one mole of 2+ ions in their gaseous state
Second ionisation equation for Mg

The stronger the attraction between the outer electrons and the positive protons in the nucleus…
The greater the ionisation energy
Factors affecting ionisation energy
Atomic radius (distance between outer electrons and nucleus)
Charge on the nucleus (the greater the amount of protons, the greater the force of attraction)
Shielding (More shells reduce the attraction)
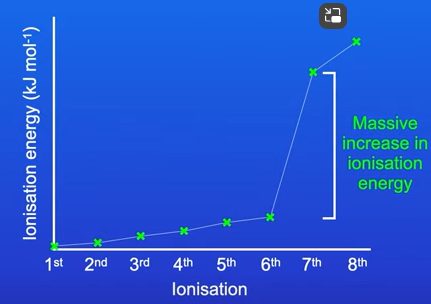
Explaining the successive ionisation energies of oxygen
For the first to six electrons there was a gradual increase in the ionisation energy
This is because all 6 electrons were removed from oxygens outer shell (low attraction between nucleus and outer electrons so little ionisation energy needed)
But the remaining 2 electrons were on the shell closest to the nucleus. Higher ionisation energy was needed to remove the 7th and 8th electron
Explain the trend for first ionisation energy down a group
FIE decreases down a group
Atomic radius increases-meaning the outer electron is further from the nucleus/weaker force of attraction
Number of energy levels increase-more shielding
Although charge on the nucleus increases its effect is cancelled by the other 2 factors
Explain the trend for FIE across a period
FIE generally increases
Nuclear charge increases- higher number of protons/stronger attraction→ more energy is needed
Strong attraction also decreases atomic radius as outer electrons are pulled closer to the nucleus
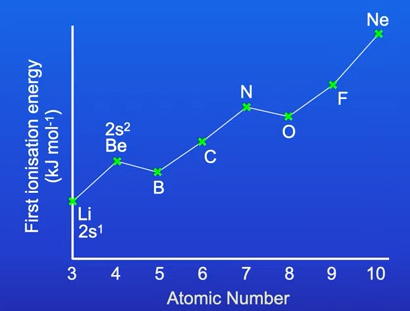
FIE of elements in period 2
In period 2 the FIE of oxygen and boron actually decreases
This is due to their electron configuration
Oxygen has a pair of electrons in its p subshell. Less energy required to remove these electrons as they repel each other
Boron has one electron in its p subshell whereas Be doesn’t have a p subshell.
It is easier to remove that one electron in boron’s p subshell as it is further away from the nucleus