chem term 1
1/141
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
142 Terms
The solutes we measure are more commonly known as
analytes
Solutes and solvents used in analytical work to assay patient specimens are called ___ grade chemicals
AR
CLRW stands for
Clinical Laboratory Reagent Water
ion exchange removes ions to produce
mineral free deionized water
what type of purification method removed bacteria, pyrogens, particulates, dissolved ions and some organics?
Distillation
What removes 95%-99% of organic compounds, bacteria, and other particulates
Reverse Osmosis
how do you produce CLRW
Pre/filters and filters
Purify
Membrane
Ion exchange
Activated carbon
To assure purity of CLRW, what is tested monthly
Microbiological
Organic impurities
Resistivity
Paticulate and colloid counts
Primary materials
Highly purified, known concentration
Secondary materials
assayed value established by reference or comparison of known materials
Certified materials
high standards with certificate of analysis
What is the glassware used to create accurate results
Class A glassware
TC
to contain
TD
To deliver
Centrifugation
the process of using centrifugal
force to separate the lighter portions from the
heavier portions.
What are the two types of centrifuges
horizontal head and fixed angle
RCF is equal too
RCF = 1.118 X 10-5 X r X rpm2
Relative centrifugal force
the amount of force to sediment particles in a centrifuge
the main thing to remeber when using a centrifuge is
balance with caps on
maintenance of a centrifuge
clean and decontaminate when things sill
check speed with a tachometer
check brushes (if present)
document when and what maintenance was done
What class of analytical weights are used to calibrate balances
Class S weights
buffer
solutions containing a weak acid or base and its equivalent salt
this solution is resistant to Ph changes
radio waves are
long
energy is inverse to
wavelength
energy is proportional to
frequency
what is not absorbed is
transmitted
beers law=
abc
what are the 4 conditions of beers law
no multiple wavelengths
no interfering sunstances
no stray light
high concentrations are diluted
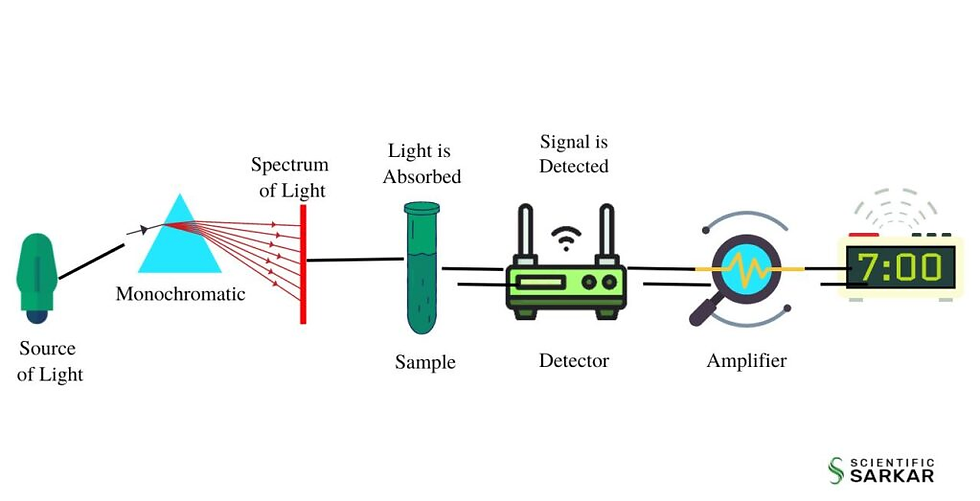
single beam spectrophotometer looks like (diagram)
UV range is
200-400nm
visible light is
400-750nm
monochromator
isolates radiant energy of a desired wavelength and excluding that of other wavelengths
what devices are used for spectral isolation
filters
prisms
diffraction gratings
bandpass
rage of a wavelength transmitted
spectral bandwidth
width of a wavelength at half the max intensity of light leaving the monochromator
the more efficient the monochromator
the narrower the bandpass
wht cuvettes are used for accurate measurements of UV
quartz
photodetectors
convert light into electrical signal
photodiode detectors
silicon
germanium
indium gallium arsenide
lead (II) sulfide
what type of lamps are used light of the ultra violet range
H+ or deuterium lamps
What type of lamp is used for the visible range
Tungsten
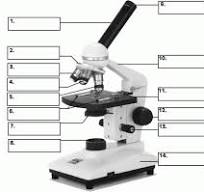
what is number 1
body tube
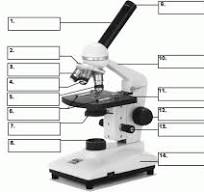
what is number 2
Revolving nose piece
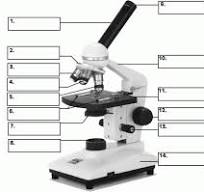
what is 3
low powered objective
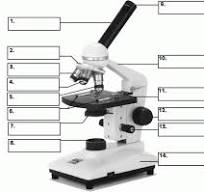
what is 4
medium powered objective
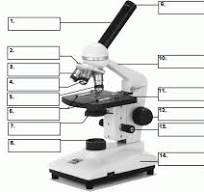
what is 5
high powered objective
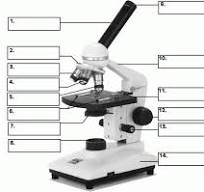
what is 6
stage clips
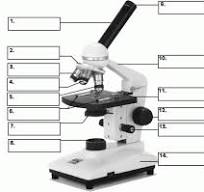
what is 7
the condenser
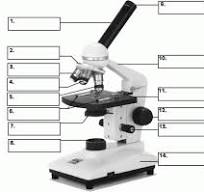
what is 8
light source
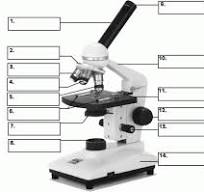
what is 9
eye piece or ocular lense
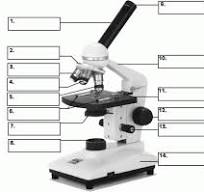
what is 10
arm
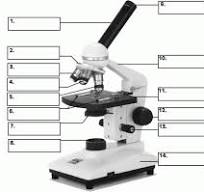
what is 11
the stage
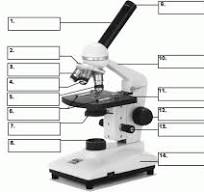
what is 12
course adjustment knob
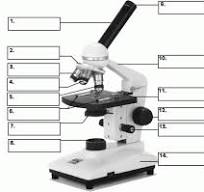
what is 13
stage adjuster
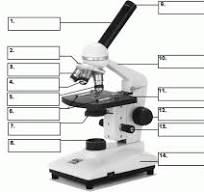
what is 14
base
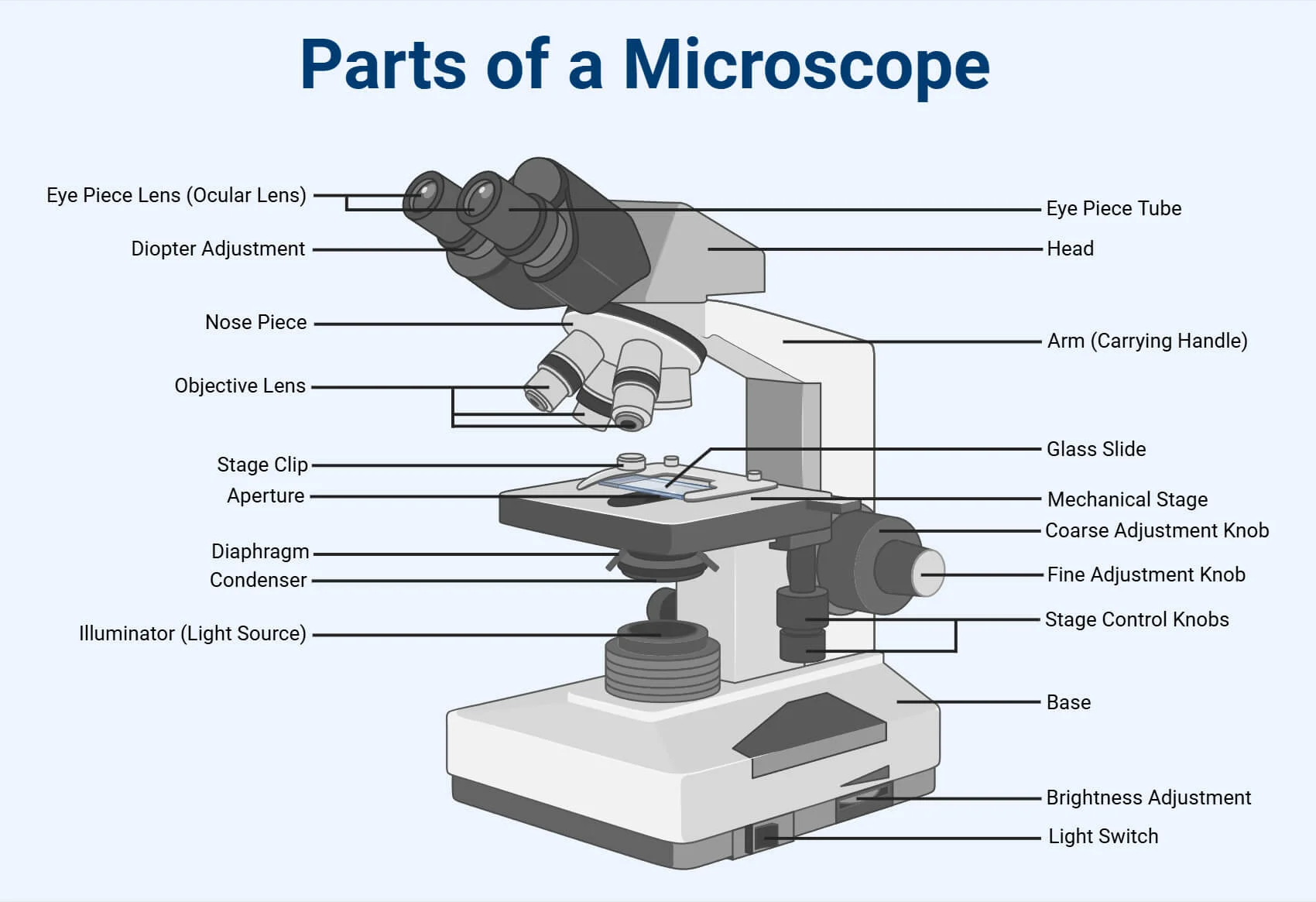
review other parts
magnification=
lens objectivex10 (40X x10= 400)
gold top and or red top
most therapeutic drugs
green top
chem test
heparin on ice
ammonia, blood gas, whole blood icals
sodium flouride
glucose testing- slows glycolysis
not used for BUN-inhibits urease
EDTA
used in heme
what is it challenging to use plasma from a EDTA for chem testing
it causes Potassium to sky rocket as potassium is in EDTA
Hemolysis causes what to increase
K+
LD
AST
AMM
Mg++
PO4
iCal
T/F Class A pipets do not need to be recalibrated by the lab
true
A self draining pipet that has the highest degree of accuracy and precision is a/an
volumetric pipet

This vessel is calibrated to hold one exact volume of liquid (TC)
Class A volumetric flask

The vessel in the image on right is designed to hold different volumes and is used to prepare reagents. what is it called?
erlenmeyer flask
centrifugal force depends on three things:
mass
speed
radius
T/F, A 1:10 dilution is the same as a 1:10 ratio
F
T/F, A 1:10 dilution is the same as a 1:9 ratio
T
serial dilutions are used for
infectious disease testing
in order for a serial dilution to be clinically significant
must see a 4-fold or 2 tube r
A= (log version)
2-log%T
at what wavelength are quartz cuvettes required
340nm
Biochromatic analysis is used to correct for
interference
What are three types of interference
hemolysis
Icterus
lipemia
What lamps have built in sources for checking wlength accuracy
H+ or deuterium lamps
What filters may be used to check for qlength calibration
holmium oxide or didymium
standard absorbing solutions are used for what
determining wavelength accuracy of instrument
Atomic absorbtion spectrophotometry detects the absorbtion of EMR by
atoms
atomic absorbtion uses a light source of
hollow cathode lamp
what kind of lamp would be used for checking copper levels
copper atom filled lamp
Fluorometry differs from spectrophotometry in what two ways
Has 2 monochromators to select excitation wavelength and the emission wavelength
detector is at right angles to the excitation beam
what are the advantages of absorption spectroscopy
more specific
more sensitive
Quenching
causes decreased readings due to absorption of emitted light by other solutes

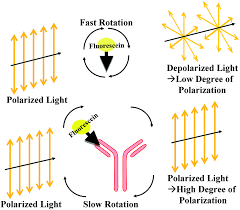
Positive FPIA
most tagged Ag unbound
reduces the amount of polarized light produced
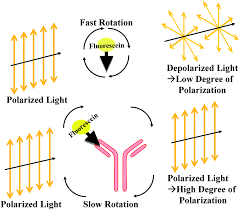
Negative FPIA
tagged Ag binds to Rgt ab
increases polarized light
Turbidimetry
measures decrease in light

nephelometry
measures scattered light (like glitter)

Quality assurance (QA)
Overview of systems and determining goals for said system and how to improve it.
what are three things in QA of a lab
pre-analytical
analytical
post-analytical
Quality control
uses control materials as a reference to determine accuracy and reliability of patients results
Mean
arthmetric average value
Median
center of all observations
mode
value greatest frequency
when the distribution is symmetrical the mode is equal to
mean and median
-2s to +2s is
95.5% of a bell curve
standard deviation is equal to
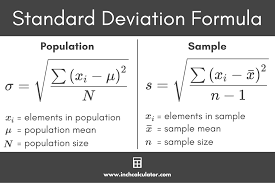
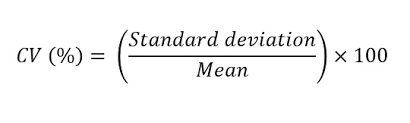
Coefficient of variation is equal to