Lecture 7: Tissue Mechanics, Mechanotransduction and Fibrosis
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
64 Terms
Mechanical properties of Body Tissues
Mechanical properties of body tissues are diverse:
Brain: Soft
Bones: Rigid and stiff
Tissues (e.g., heart, skin): Must deform to enable movement
Measurement of Mechanical Properties
Plotted on a scale - Measures on an Elasticity Scale (kPa):
Brain and bone marrow: Soft (1 kPa)
Non-calcified stiff tissue: 50 kPa
Calcified bones with minerals: Stiff, non-deformable (100 kPa)
The Young’s Modulus (E)
It is a measure of stiffness or elasticity
It is a measure of how much is required to deform a tissue
The large the number, the more force required to deform it
Key Concept of Mechnaincal Properties
Mechanical properties are matched to the function of tissue
This gives rise to the diversity in properties e.g. stiff bones to carry weight, robust skin to form a protective barrier
Usefulness of Brain being a Soft Tissue
The soft nature of brain tissue is beneficial in development
It allows the remodelling of tissues in the presence of the skull, which offers a source of protection
Tissue Composition
Composed of cells and extracellular matrix
Extracellular Matrix
It is a 3D network of extracellular macromolecules found in multicellular organisms
It is the main composition of humans → occurs for over 50% of dry mass
Most of this is collagen (most abundant protein in the body)
It defines the mechanical properties of our tissue
Produced by cells and is maintained throughout life
Decellularisation of Tissues
Organs can have cells washed out using sufactant, leaving behind a collagenous structure and mechanical properties maintained in the tissues, just as if the cells were present
The cells are removed → it demonstrates the matrix’s responsiblity for the mechanical properties measured
How Do Cells Communicate With the Surroundings
Cells and ECM exist together in all tissues
Cells communicate with the surroundings through contact with the ECM, where they take signals (biochemical and through mechanotransduction) and respond
The signalling processes in response to signals from the surroundings can explain the different functions of cells with similar genetic material
How Can Cells Change Their Environment
Secreting new proteis
Remodelling proteins and molecules in the environemnt
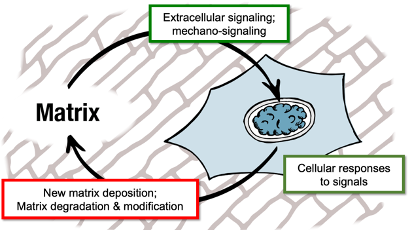
ECM-Cell Response Loop In Development
Extracellular or mechanic signal detected by cell
This triggers a cellular response
Cellular response includes: new matrix deposition, matrix degradation and modification
Homeostatic System
Systems that actively regulated to maintain a steady state
In healthy adult bodies, tissues are maintained in a steady state even when challenged by the environment – constantly regenerated and repaired through self correction and regulation via feedback loops
Disease processes can disrupt this balance
Cell Morphology
The shape and appearance of a cell
It can be determined experimentally by placing cells of a substrate and controlling the mechanical properties of a substrate e.g. hydrogel
Hydrogel
Synthetic polymer gel, often polyacrylamide on which cells can be cultured
Cells can detect the stiffness of the substrate environment and change their shape in response
It can be made softer of stiffer by varying the density of cross-links
softer gels deform more
stiffer gels deform less
Cell Morphology Studies: Stiff Hydrogel
Cells are larger and spread out more
Occurs in cartilage (similar stiffness)
Cell Morphology Studies: Soft Hydrogel
Cells are smaller and balled up
Occurs in bone marrow → similar stiffness
Key Concepts of Tissue Stiffness and Cell Morphology
Cells spread out more of stiffer substrates
The cells get bigger and the nuclei is pulled flat
The shape of the cells changes
Contractility
How hard the cells are trying to deform their surroundings
Cells hold onto their surroundings and pull against them
Cells detect stiffness by deforming their surroundings → only detect mechanical properties by interacting with the surroundings
Key Concept of Tissue Stiffness Cellular Contractility
Cells pull harder and are more contractile on stiffer substrates
Cell cultured on a stiff and strong spring
The stiffer the spring the harder the cells pull against this
This reaches a plateau, where the cell only produces a certain amount of force → reaches a max level due to the amount of machinery present
2 phase behavoiur
Key Concept of Tissue Stiffness and Proliferation
Cells grow faster on stiffer substrates
Seen in 3 different types of cells
Vascular smooth muscle cells
Mammary tissue
Fibroblasts
Key Concept of Tissue Stiffness and Apoptosis
Apoptosis is slower on stiffer substrates
In stiffer environments, cells proliferate faster but apoptoses slower → greater rate of cell population increase
Durotaxis
Movement of cells on different substrates of stiffness
Investigates using a substrate on a gell with a gradient of stiffness
Cell crawls towards the stiff environment
Cell migration influenced by a gradient of mechanical properties
Key Concept of Tissue Stiffness and Durotaxis
Cells migrate towards stiffer regions/ environments
Differentiation
The commitment of a cell to a particular lineage
Stem cells can have a n.o of different fates
Key Concept of Tissue Stiffness and Differentiation
Stiffness can direct cell fate.
Choses a fate influenced by the mechanical properties of its surroundings
E.g. Mesenchymal cells
Mesenchymal Stem Cells In Soft Substrates
Drives differentiation of MSC to soft tissue types e.g. fat
They become adipogenic → forms fat tissue
Mesenchymal Stem Cells In Stiff Substrates
Drives differentiation of MSC to stiff tissue types e.g. bone
They become osteogenic → form bone
YAP - a TF favours osteogenesis in these cells
Cell Behaviour Influenced by Mechanical Signals
Cell morphology (e.g., spreading and shape)
Contractility (how hard cells pull on their surroundings)
Propagation rate and apoptosis
Cell movement (durotaxis)
Differentiation (commitment to lineage)
Mechanotransduction
The conversion of mechanical input into a biochemical signal
Allows the cells to respond to mechanical signal through a series of signalling pathways
How Do Cells Detect Stiffness
By deforming their surroundings
Must apply forces and have a feedback mechanism to detect whether the surroundings have been deformed
Mechanisms Required By Cells
Force generation (actomyosin cytoskeleton contraction)
Force transmission (cytoskeleton- structural proteins that make up and form the mechanical backbone of the cell)
Mechanosensing (conversion into biochemical signals to activate a pathway to modify cell behaviour)
This may be done by protein modification or activation of a transcription programme
Adhesion Complex
A system of proteins that form at the cell membrane and links the cell to its surroundings
It acts as a bridge between the ECM where the cells is attached to and the cytoskeleton
It gives structure to the cell
It consists of
Integrins
Actin
Myosin
Talin
Integrins
Membrane proteins that form focal adhesion complexes that tether the cytoskeleton to the matrix.
Binds to receptors that span the cell membrane
Actin
Polymeric filaments and a major component of the cytoskeleton;
They form the growth of filaments that drives cell spreading – push out at the edge of the cell.
Myosins
Molecular motors proteins that pull against actin filaments, causing contractility.
Talin
A protein that deforms when pulled on, activating a signalling cascade (conversion into biochemical signal).
Provides mechanosensing as it has lots of domains that unfold to reveal cryptic sites in response to ‘pulling’ that can interact with other proteins
Cell Crawling
Leading edge Actin filaments polymerise and extend, pushing the cell forward.
Retrograde flow: Myosin pushes filaments back, dissolving them and allowing the cell to pull itself forward and away from its surroundings.
Relationship between focal adhesion, cytoskeletal tension, and cellular mechanics
Cells pull on their surroundings using actin polymerisation at the leading cell edge and myosin-II (non-muscle myosin 2A).
Soft surroundings deform under the cell’s pull.
Stiff surroundings don’t deform, but mechanosensing proteins (like talin) within the cell do, revealing cryptic binding sites.
This allows vinculin to bind, activating a signalling pathway that involves MAPK and RhoA.
Signalling leads to more actin and myosin production, increasing tension on the substrate → pulls harder against substrate
Focal Adhesion Complex and Cytoskeletal Tension: Stiff Substrate
Stiff substrate activates signalling proteins that increase actin and myosin production.
Actin polymerises, pushing out at the edge of the cell, leading to cell spreading.
Myosin pulls harder against fibres, increasing cell contractility.
Key signalling molecules:
MAPK (Mitogen-Activated Protein Kinase)
RhoA (Transforming Protein RhoA)
These molecules drive increased actin and myosin production, causing the cell to generate more force and spread further.
Ion Channels
Protein complexes that perforate the cell membrane to form pores
They allow the movement of substances in and out of cells
Transient Receptor Potential Vanilloid 4 (TRPV4) Channel
A mechanosensitive ion channel
When the cell membrane is under tension it is transferred to these ion channels, causing them to open
It aqllows ions to enter, changing the intracellular concentration, and causes the activation of a signalling pathway – conversion of signal occurs
Conversion Process of mechanical input (membrane tension) to Chemical signal (ion concentration)
Mechanotransimission From Outside the Cell To Nuclues
In the nucleus, chromatin can be remodelled to affect its interaction with transcription factor, transcription and protein expression
Changes outside in surroundings can be transmitted through the cell and through the cytoskeleton to the nucleus and transferred to chromatin, changing its organisation
Changes in chromatin organisation cause dfferent sites to be activated and accessible → can turn on/off different combinations of genes
Meadted by the transmission system: focal adhesion complex, actin and myosin within the cytoskeleton
LINC Complex
A complex of proteins that links the cytoskeleton in the cytosol to the nucleus
It is made up of nespurin → binds to sun proteins
Sun proteins cross the nuclear envelop and tether to laminin
Laminin
A network of proteins that forms a cargo net structure on the inside of the nuclear envelope, containing chromatin
It also provides mechanical robustness to the nucleus
Force Transmission: Cytoskeleton → Nucleus
Force is applied to the cytoskeleton, where actin then transmits this to the nesprins proteins which bind to SUN proteins and transmitting forces through to the lamina, inside the nucleus and changes the organisation of chromatin, regulating its activity
Disruption of LINC complex
Due to point mutations in the laminins, nesprins or sun proteins that affect the transmission of force
It blocks the mechanotransmission to the nuclues
YAP1L:(“yes-associated protein 1”)
A transcription factor involved in the development, and differentiation of cancer
Its behaviour is regulated by its localisation inside the nucleus → it is a transcriptional activator and can interact with its target site
When localised in the nucleus it drives osteogenic differentiation
If moved out of the nucleus, it is inactivated → no longer interacts with DNA
Regulation of TF by moving it in/out nucleus
Mechanoregulation of Transcription Factors
It allows control of specific genetic programs and pathways; movement driven by mechanical properties and input → due to control of factors affecting certain genes
Translocation can be mechanically regulated by the properties of the environment
Cell in soft environment: YAP outside the nucleus
Cell in stiff environment: YAP inside the nucleus - drives oestogeneis
How Do Cells Respond to Physical Properties of Their Environment
Mechanotransduction signalling pathways allow cells to detect mechanical properties of their environments and respond:
Signaling from focal adhesion complexes (MAPK and RhoA)
Mechanosensitive ion channels (e.g., TRPV1)
Transmission of force to the nucleus (LINC complex)
Translocation of transcription factors (e.g., YAP1)
Fibrosis
Occurs in response to a misregulation of feedback and loss of homeostasis causing cells to deposit too much matrix
The mechanical properties are altered
Tissues become more collagenous and stiffer → changes the environment and biochemical signals with the tissue deviating from mechanical homestatsis
Mechanical properties no longer matched to function
Fibroblasts
Cells responsible for synthesising extracellular matrix, such as collagen.
They don’t form a continuous layer and can move around when cultured in 2D
Cells can move towards site of injury (durotaxis → move to scar)
Necessary for wound healing
Myofibroblasts
Activated fibroblasts in response to injury
These cells are more contractile, pulling harder on their environment and secrete more ECM, contributing to fibrosis (excess matrix)
They are formed by mechanical stimulation e.g. in response to a stiff environment and by chemical signals
e.g. TFGB-1 → cause differentiation of fibroblasts into myofibroblasts
Examples of Fibrotitc Diseases
Severe atherosclerosis (thickening of blood vessel walls) of the aorta. – constricts blood flow
COPD (chronic obstructive pulmonary disease) lung tissue – blocks gas exchange
Excessive matrix prevents the correct function
Role of collagen and matrix in tissue repair
Collagen and matrix are produced to fix a wound.
Importance of Scaring
It can be healthy because it helps wounds heal and prevents further damage by forming a protective layer.
But it must eventually be resolved by removing excess matrix and collagen to return to the healthy starting point.
excess scar tissue can lead to loss of function
Excessive Scarring Fibrosis
Tissue doesn’t return to healthy tissue function due to the presence of excess matrix and scar tissue
The environment becomes too contractile and restricts movement, leading to pathology
Fibrotic Response
Excessive scarring is not resolved
Dysregulation of the ECM causes changes in the mechanical environment as the scar tissue is stiff
An immune response is generated leading to the infiltration of immune cells
These factors disrupt tissue function, causing further damage; and resulting in a second feedback loop of a fibrotic response
Healthy Lungs
Air passes through bronchioles into the alveoli which have thin membranes to allow gas exchange
Idiopathic Pulmonary Fibrosis (IPF)
Fibrosis occurs in the alveoli, resulting in the presence of excess matrix that prevents gas exchange and results in suffocation → low removal and uptake of CO2 and O2
Not a rare disease → affects 100,000 people aged 14-43
Affects men more than women
Diagnosed by occluded x-rays – matrix build-up or blocking up of the lungs – white trace
Average diagnosis time: 1-2 years after symptom onset
Fatal - 50% mortality rate within 2-3 years of diagnosis
Symptoms of Idiopathic Pulmonary Fibrosis
Common
Shortness of breath
Chronic dry cough
Finger clubbing due to growth factor signalling produced by the scar tissue
Occasional
Fatigue
Weakness
Weight loss
Cause of IPF
Idiopathic - unknown but risk factors include
Smoking
Environmental exposures
Chronic viral infection
Abnormal acid reflux
Family history
Ageing – large risk factor for fibrotic disease
Characterisation of IPF
A group of myofibroblasts – push into healthy tissue, leaving behind scare tissue
Myofibroblasts at the leading edge of the disease region produce too much matrix
Mass Spectrometry Proteomics
Used to analyse cross-sections of tissue and ECM composition to identify proteins present
Contains more Collagen I than normal → typical of scarring
It builds up in scar tissue, causing stiffness and blocking gas exchange → contributes to disease phenotype
Pathophysiology of Idiopathic Pulmonary Fibrosis (IPF)
IPF involves tissue insult → causes scarring and dysregulation of the extracellular matrix.
This leads to collagen-I production, altering tissue mechanics and the formation of a fibrotic front, disrupting alveolar function.
Immune response causes inflammation and thickening of alveolar walls resulting in impaired gas exchange and disrupted tissue function, leading to organ failure.
A positive feedback loop ensues, where ongoing injury promotes further fibrosis. → continuing injury that drives the fibrotic response