Addition, Reduction, & Oxidation Reactions
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
38 Terms
Mechanism I
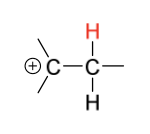
1) Proton (H+) adds to alkene to form C+
2) Nucleophile adds to C+
stereoselectivity: syn + anti products
regioselectivity: Markovnikov (more stable C+)
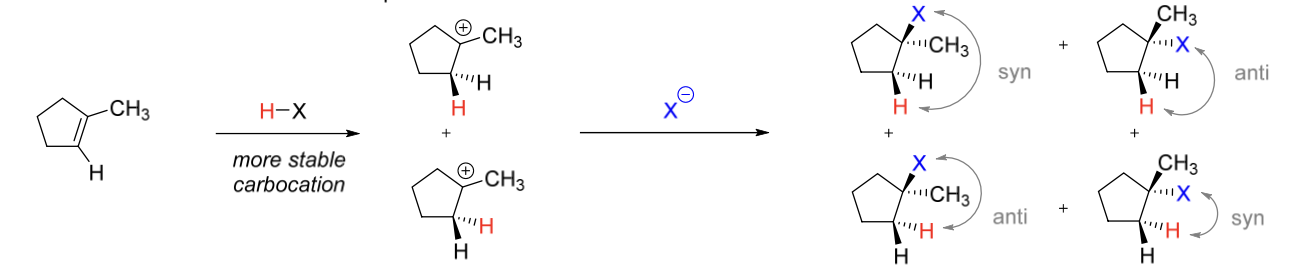
Mechanism I stereoselectivity
syn + anti products
Mechanism I regioselectivity
Markovnik (more stable C+)
Mechanism II
1) Electrophile (E+) adds to form 3-membered cyclic intermediate
2) Nucleophile attacks the cyclic intermediate to open it and form the final product
stereoselectivity: anti
regioselectivity: X- adds to side that best stabilizes to partial (+)

Mechanism II stereoselectivity
anti products
Mechanism II stereoselectivity
X- adds to side that best stabilizes partial (+)
Mechanism III
two groups that are connected to each other add simultaneously to alkene
stereoselectivity: syn
regioselectivity: matching partial (+) or (-) or based on size
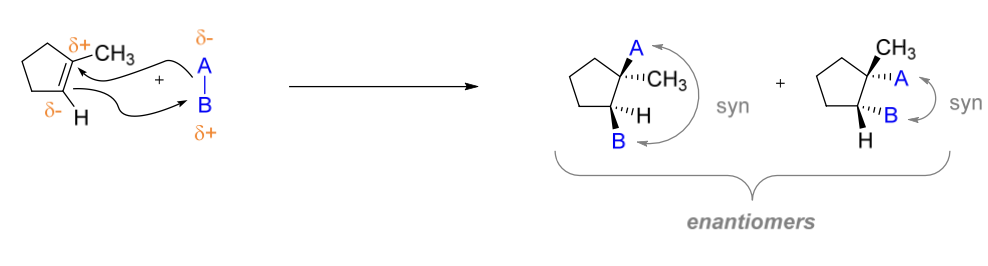
Mechanism III stereoselectivity
syn products
Mechanism III regioselectivity
matching partial (+) or (-) or based on size
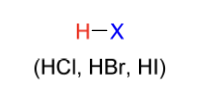
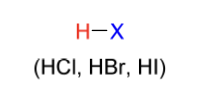
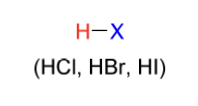
hydrohalogenation electrophile
H-X
(HCl, HBr, HI)
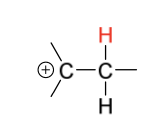
hydrohalogenation intermediate + reagent in second step
X-
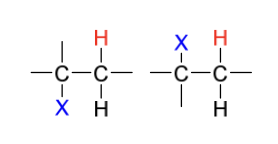
hydrohalogenation product(s)
Mechanism I
syn + anti
Markovnikov
hydration electrophile
H2O + catalytic acid
ROH + catalytic acid
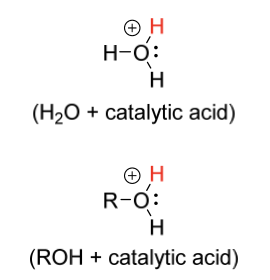
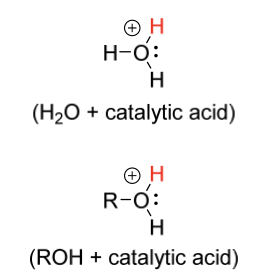
hydration intermediate + reagent in 2nd step
H2O
ROH
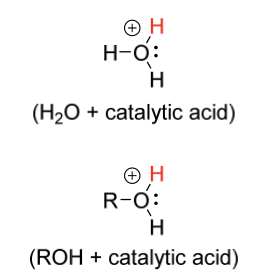
hydration product(s)
Mechanism I
syn + anti
Markovnikov
halogenation electrophile
X2
(Br2, Cl2)
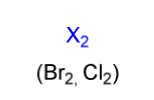
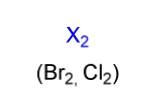
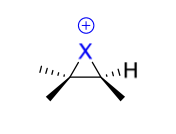
halogenation intermediate + reagent in 2nd step
X-
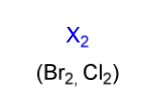
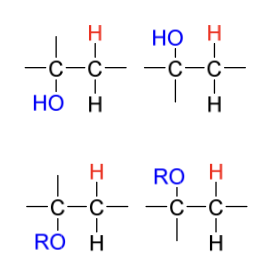
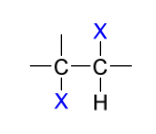
halogenation product(s)
Mechanism
anti addition
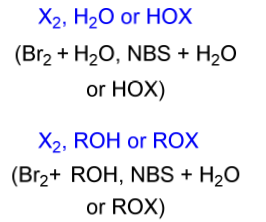
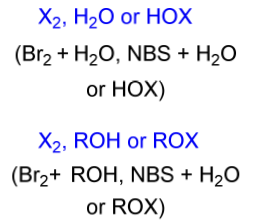
halohydrin electrophile
X2, H2O or HOX
X2, ROH or ROX
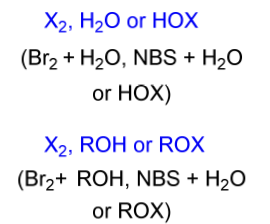
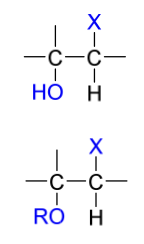
halohydrin intermediate + reagent in 2nd step
H2O
ROH
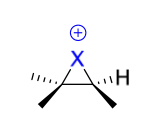
halohydrin product(s)
Mechanism II
anti addition
addition of “OH”/”OR” to more substituted C
hydroboration electrophile
1) BH3 or B2H6
2) H2O2, NaOH
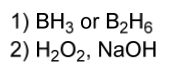
hydroboration intermediate + reagent in 2nd step
H2O2
NaOH
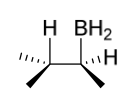
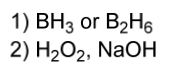
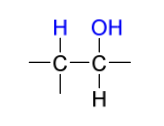
hydroboration product(s)
Mechanism III
syn additions
anti Markovnikov
dihydroxylation reagents
OsO4
KMnO4
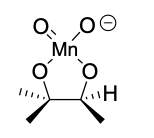
OsO4 (dihydroxylation) intermediate + reagents in 2nd step
Na2SO3
H2O
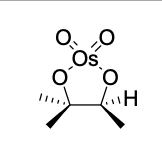
OsO4 (dihydroxylation) products
Mechanism III
syn addition
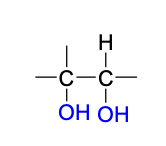
KMnO4 (dihydroxylation) intermediate + reagents in 2nd step
NaOH
H2O
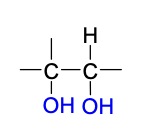
KMnO4 (dihydroxylation) products
Mechanism III
syn addition
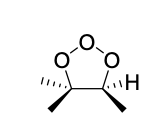
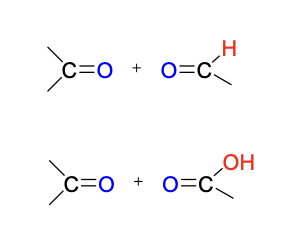
ozonolysis reagent
O3
ozonolysis intermediate + reagents in 2nd step
reductive workup: Zn, H2O OR (H3C)2S
oxidative workup: H2O2
ozonolysis product(s)
reductive (top) & oxidative (bottom)
Mechanism III
no stereoselectivity
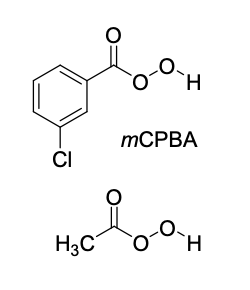
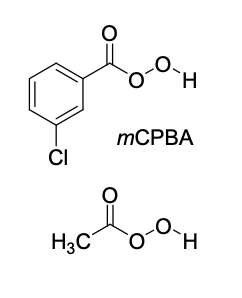
epoxidation
mCPBA
no second step!
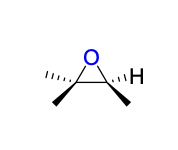
epoxidation (mCPBA) product(s)
Mechanism III
syn addition
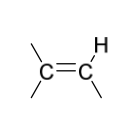
reduction reagents to create syn addition
H2
Pd, Pd/C, PtO2, or Ni
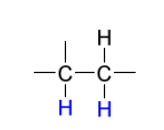
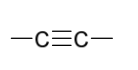
reduction reagents to create syn addition
H2
Pd, Pd/C, PtO2, or Ni
Mechanism III
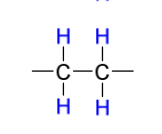
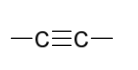
reduction reagents to create syn addition (with poisoned catalyst)
H2
Pd-CaCO3, OR Pd-BaSO4
quinoline OR PbO
Mechanism III
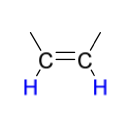
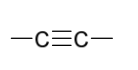
reduction reagents to create anti addition
Na OR Li
in NH3