Making salts
1/13
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
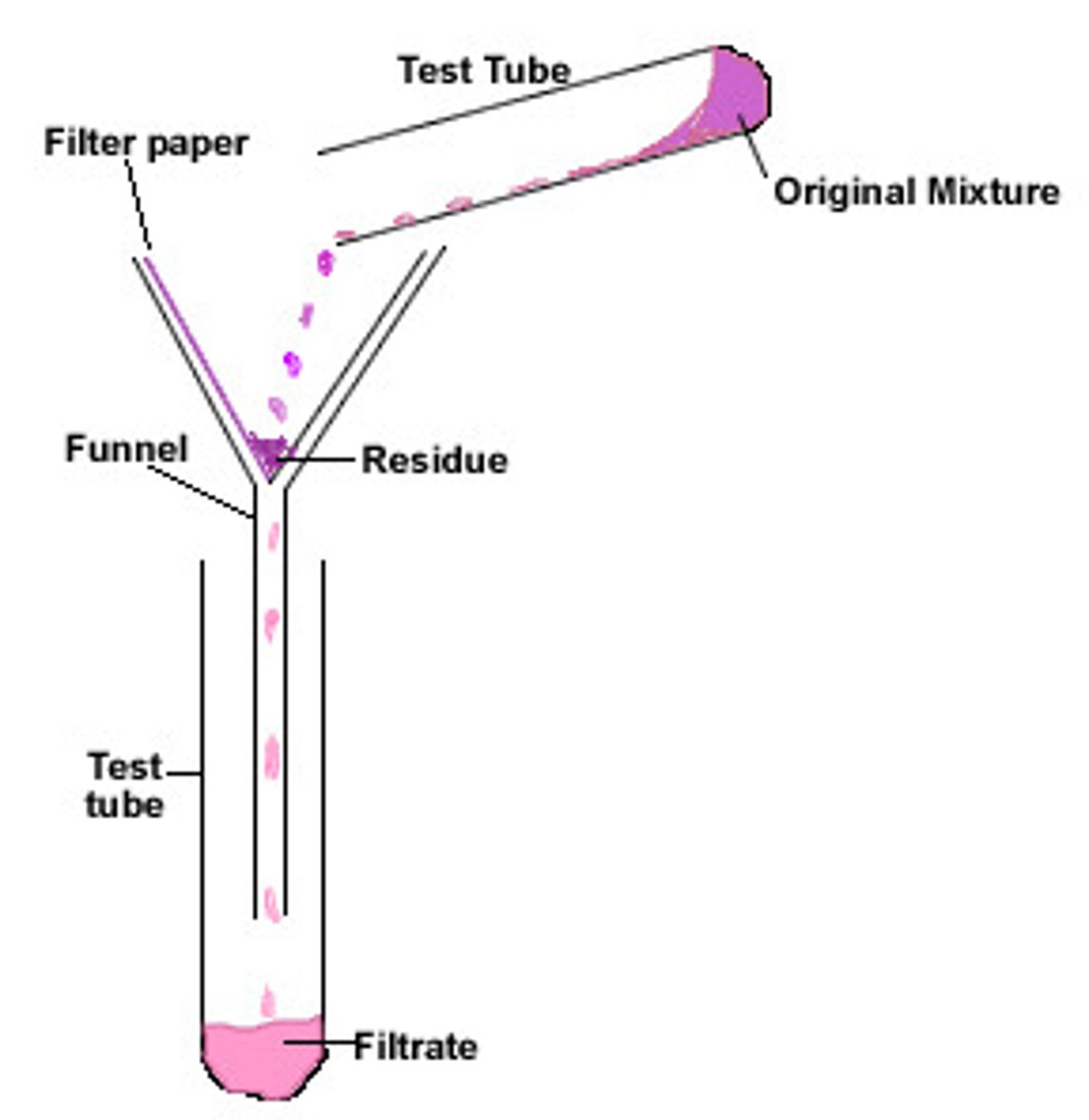
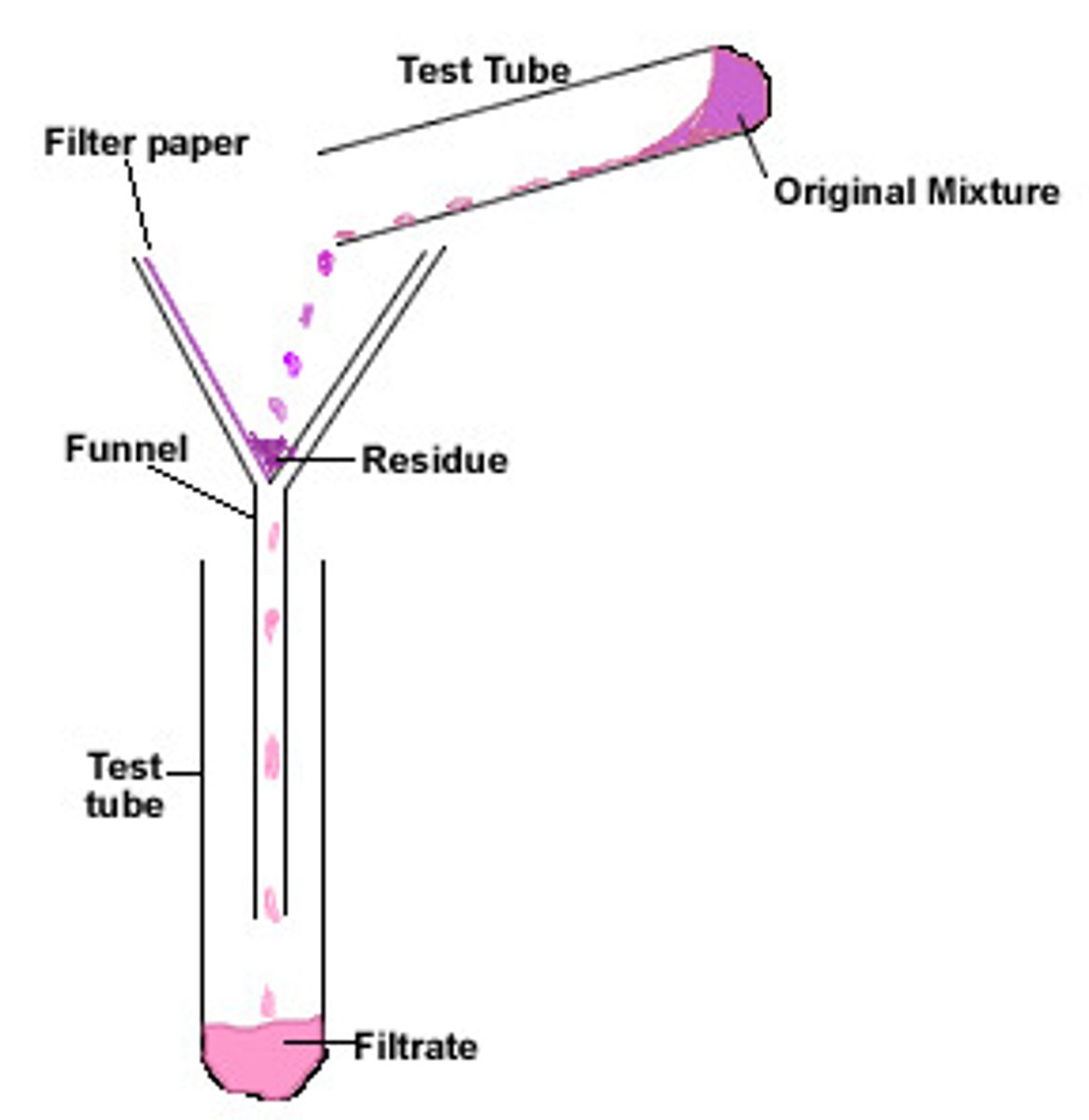
Filtration
Separates insoluble solid from a liquid
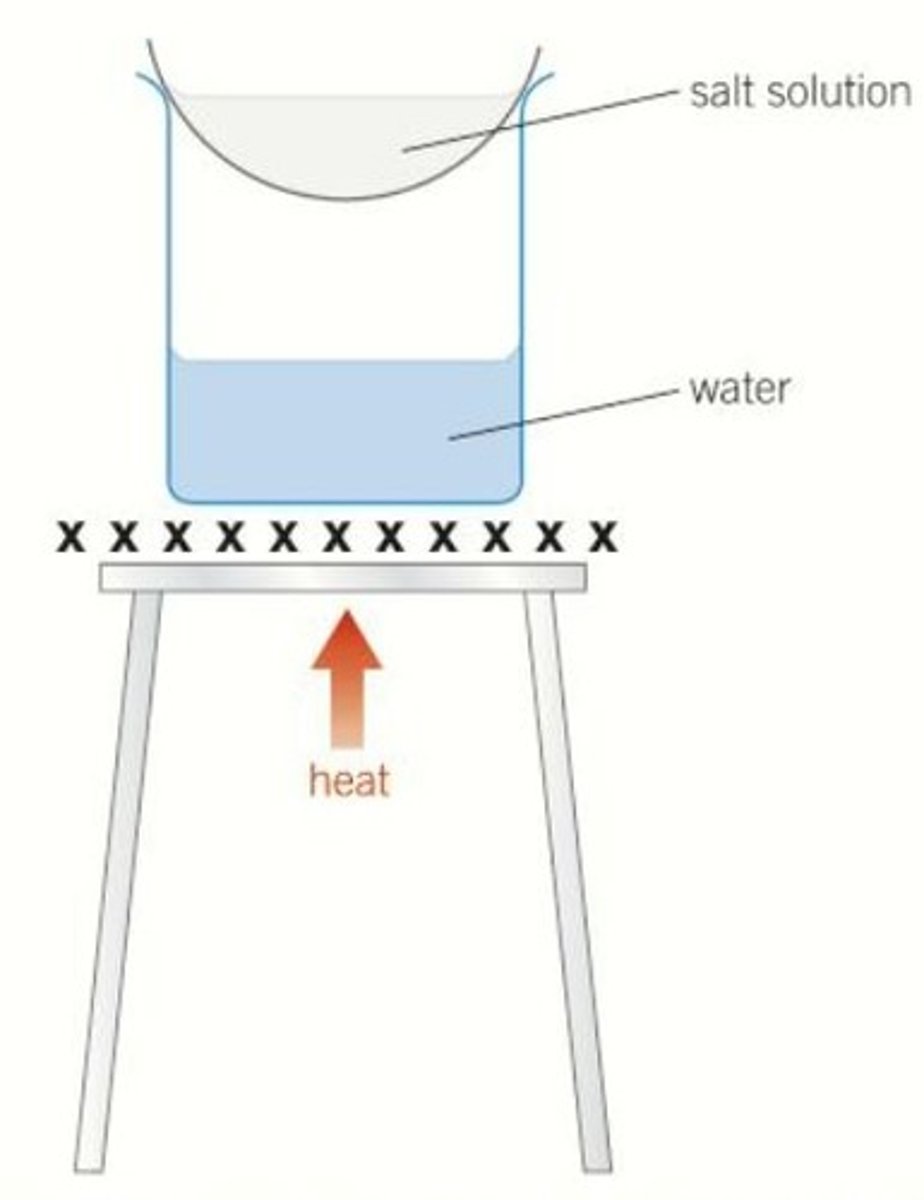
Crystallisation
The formation of crystals (as solvent evaporates from solution)
Filtrate
Liquid that has passed through a filter
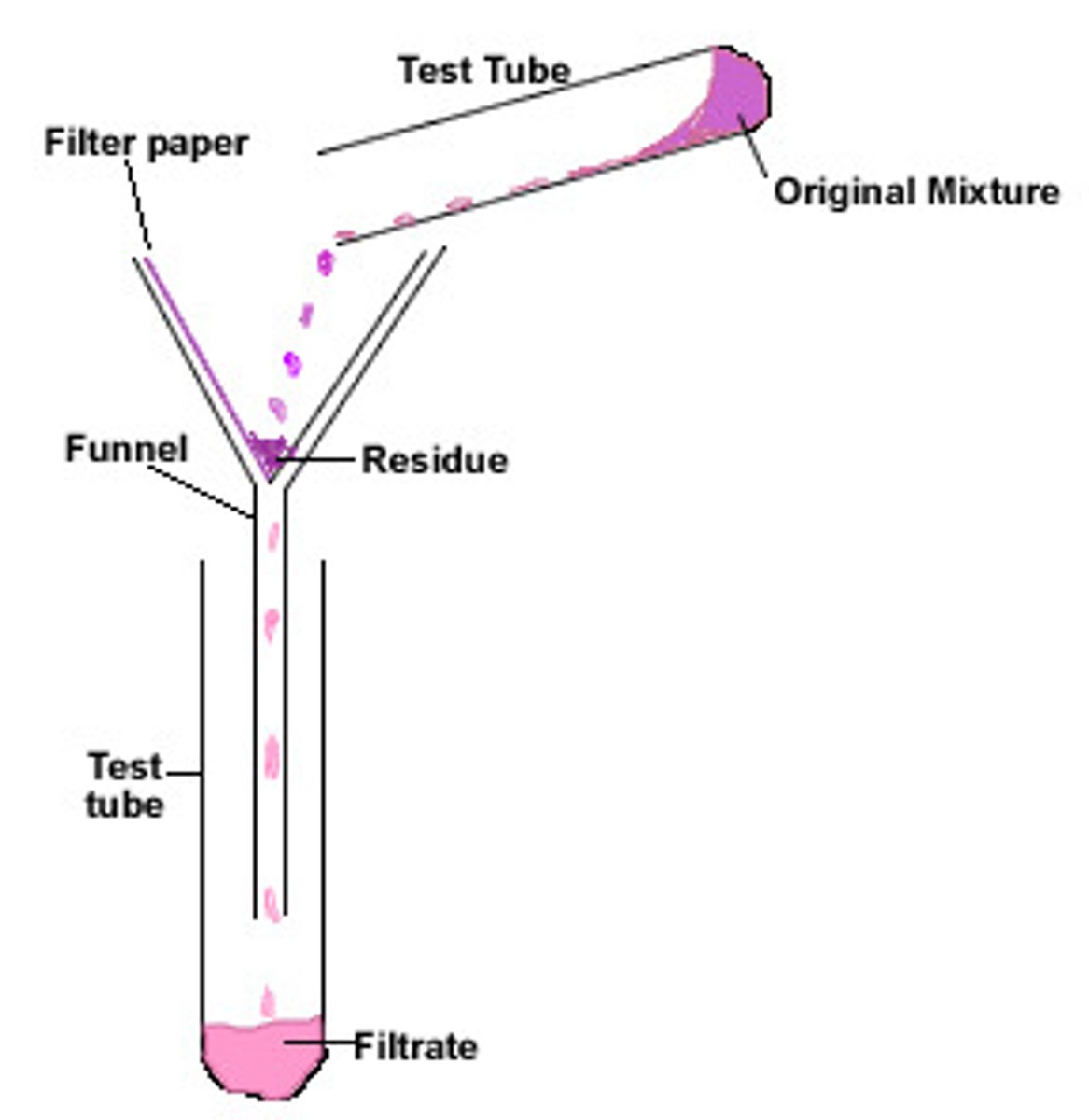
Residue
The insoluble solid left in the filter paper
Advantage of heating a reaction
Speeds the reaction up
Making salt from acid+base
Reacting an excess of insoluble base with an acid, filter off excess base
Making a salt from acid+metal
React an excess of metal with an acid, filter off excess metal
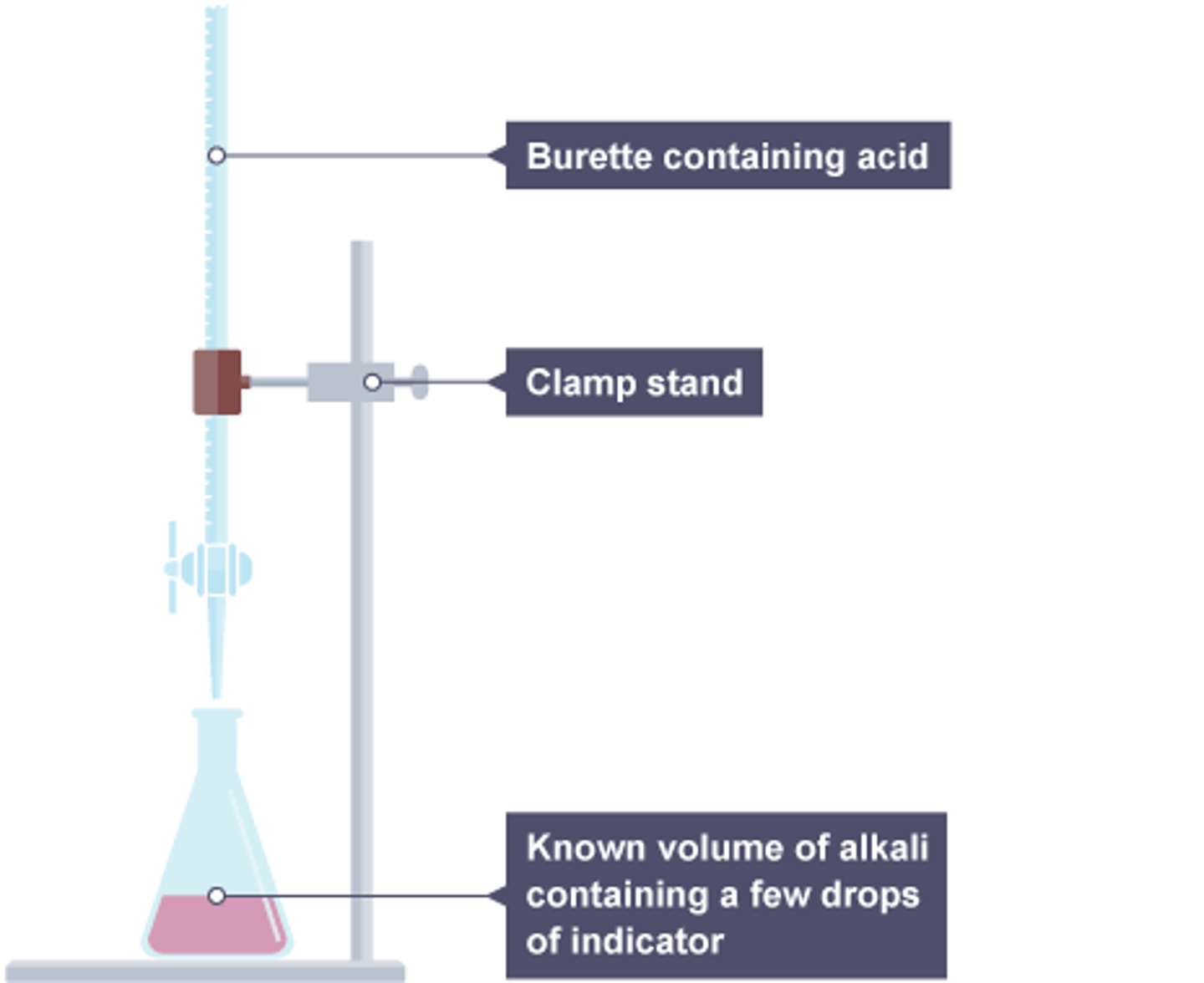
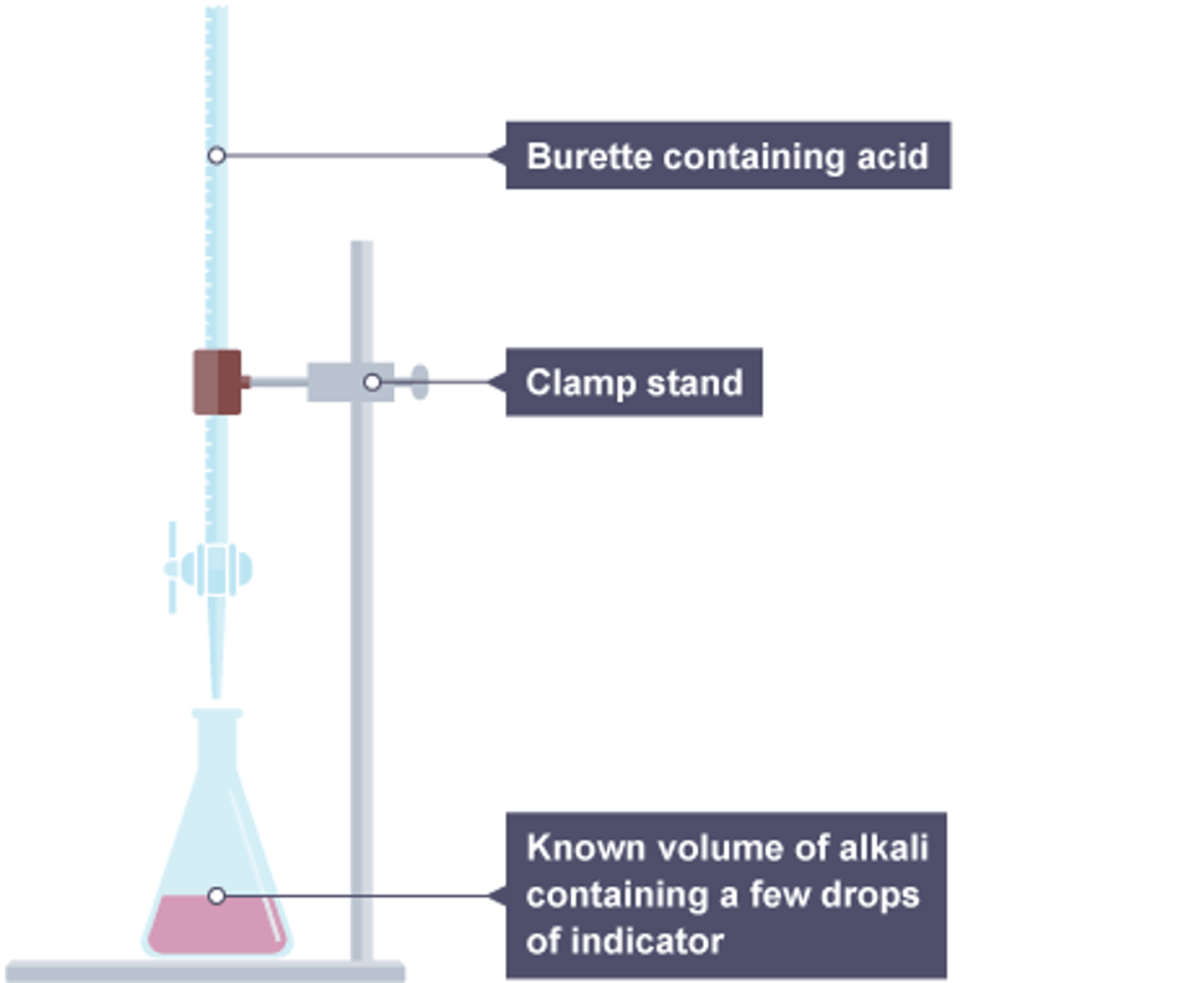
Making salt from acid+alkali
Use titration with an indicator to find the amount alkali to neutralise the acid, repeat without indicator to make a pure sample
Making pure dry crystals
Crystallisation then pat crystals dry between two pieces of filter paper
Advantage of a water bath
Heats gently, allowing water to evaporate slowly
Indicator
A compound that changes color in the presence of an acid or a base
Pipette
A glass or plastic tube used to accurately measure liquid
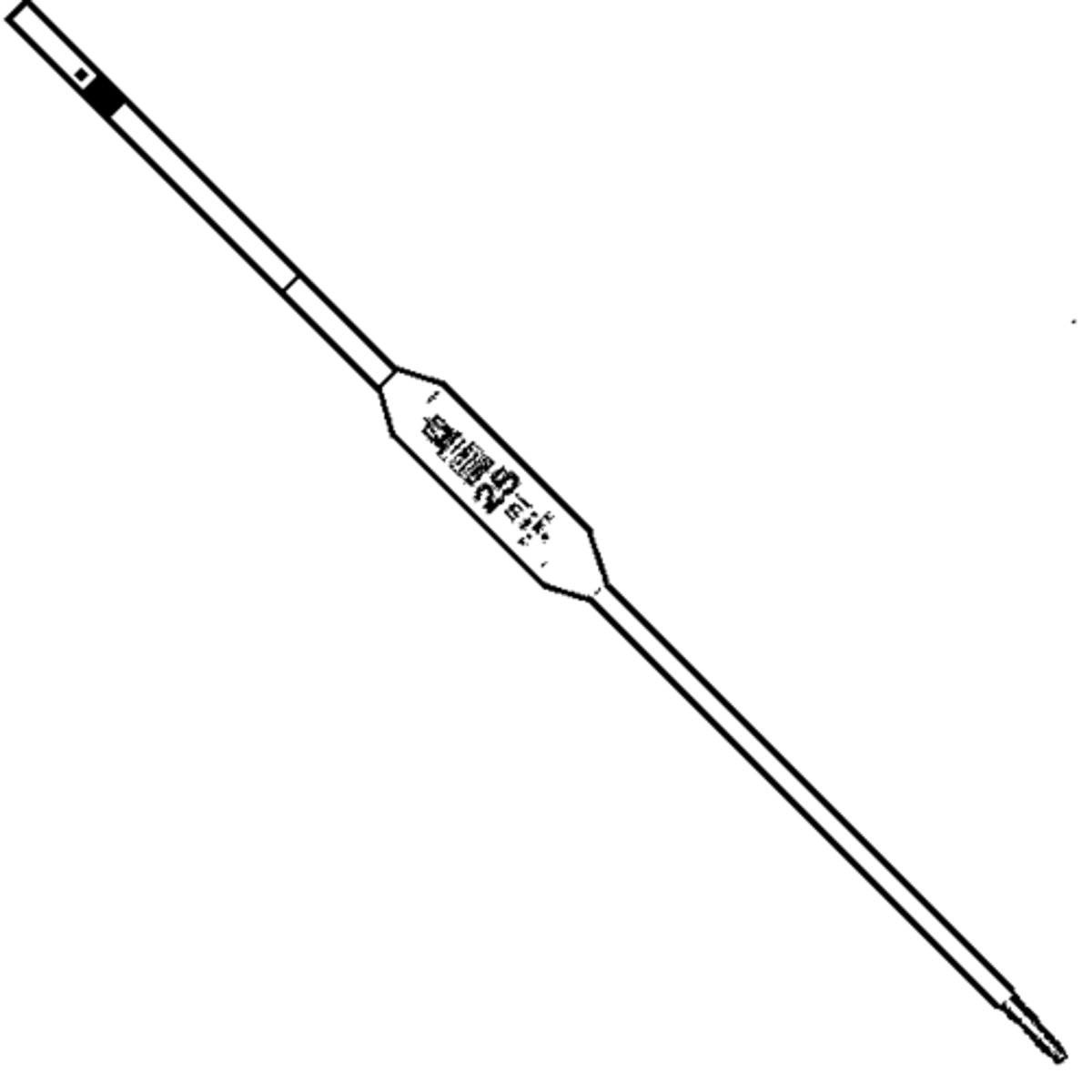
Burette
A graduated glass tube with a tap at one end, for delivering known volumes of a liquid

Making salt from an acid and an alkali
Use a titration to find the amounts of acid and alkali, repeat without indicator, evaporate water