BCHM112: Reaction Mechanisms
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
45 Terms
what does arrow pushing mean
what type of arrows are used for this
using curly arrows to explain what is occurring in a reaction, by showing movement of electrons / electron density / electron pairs (therefore formation and breaking of bonds - made of electrons)
what are the 3 main rules of arrow pushing
curly arrows start at an electron pair
curly arrows finish where electrons end up
must follow the rules of bonding / valence, with regards to the atoms and where electron pairs end up (must be possible and real)
must generally make sense!
define heterolytic bond cleavage
how would you use curly arrows to represent this
a simple bond breaking, a movement of a shared electron pair in the bond, to one of the atoms (Depends on which one needs them for valence rules)
this breaks the bond, and forms a positively and negatively charged atom (one has electrons removed, one has electrons gained) - must maintain charge balance if elementary
this would look like an arrow from the bond, towards the atom that gains the electron pair
define a nucleophile
what is the key characteristic they have
provide an example
something electron rich, with either a slight or full negative charge, or excess electron density
e.g. electronegative atom with a negative dipole in a polar molecule, an anion, a double bond, an atom with a lone pair, H-electropositive atom
this means it wants to balance this negative charge, so it seeks positive charge to attack (nucleophile = nucleus (positive) loving)
e.g. Br-, CH2=CH2, H-:O:-H
define an electrophile
what is its key characteristic
provide an example
an atom with a slight or full positive charge
e.g. a cation, or an atom with a positive dipole in a polar molecule
e.g. Br+, H, C-electronegative elements (the C would be the EP)
how does bond formation occur considering electrophiles and nucleophiles
bond formation occurs when something electron rich (Nu) attacks something electron poor (EP), as it wants to balance its negative charge, so it seeks positive charge
this forms a bond, balancing the charges, as an electron pair is transferred from the Nu → EP
how do the labels electrophile and nucleophile exist in relation to eachother
reactions in general require a Nu and EP interacting, to break and form bonds, therefore if one species is the Nu, the other must be the EP
also, Nu and EP arent the same for each reaction, one species role may change between reactions, as these labels consider Nu as electron rich IN RELATION to the other species, so they both may be electron rich, but the lesser one would be the EP
distinguish between concerted and stepwise reactions
how do these differ in terms of curly arrows
concerted reactions occur all at once, so curly arrows will show everything that happens in the reaction at once, and there will be no intermediates
stepwise reactions occur in individual steps, in a series for the overall reaction, so arrow pushing may create transition states that aren’t correct in terms of valence, and intermediate species that go on to carry out the next step so arent formed as products in the reaction
name and distinguish between the 3 main types of organic reactions
(substitution) a group on the molecule swaps with something else, the prexisting group is released
(addition) 2 groups join onto a molecule, as the pi double bond in an alkene breaks (we lose the pi bond, we gain 2 groups)
(elimintation) the opposite of adition, we lose 2 groups off a molecule as a double (pi) bond is formed
how does a general nucleophilic substitution reaction occur
can occur in one or multiple steps
a negative group (nucleophile) attacks a positive group (ep), forming a bond (sharing its excess electron density) - it joins the molecule
this EP is then unstable, as the rules of valence arent followed, so an excess electron pair is placed onto one of the bonded atoms, breaking the bond and resulting in a leaving group leaving the molecule
therefore substitution has occurred
what makes a leaving group good / realistic to occur in reaction
must consider electronegativity of the atom, if electronegative it makes the C of the C-X more positive (polarises the bond, pulls electron density), so makes the C a better EP likelier to be attacked
must consider bond strength, the weaker the bond the easier to break and leave the molecule
must consider the stability of the leaving group at the end, as an anion in solution
this is because, forming an unstable anion increases energy in the system, requires energy to form - thus is not favorable or likely to occur
so again consider EN, if more EN more stable to hold this negative charge
consider size, if larger it has more space to spread this negative charge (more stable)
consider strength (pKa) of conjugate acid, the stronger the more stable the anion in solution (as this would make the anion a weak base, more stable)
what makes halides good leaving groups?
which halides are best / worst and why
they are electronegative when C-X, so they polarise this bond by pulling electron density, making C more positive and likelier to be attacked (F>Cl>Br>I) - I is the least EN so is unreactive initially, but once started it becomes a good LG
bond strength of C-X is (F>Cl>Br>I), F being the highest makes it a bad leaving group as the bond is too strong, however the other bonds are weak enough to be broken so make good LGs
strength (pKa) of conjugate acids, HCl, HBr, HI = strong acids, therefore as anions are stable (Weak bases), while HF is weak so are strong bases in solution, thus unstable anions
therefore F is a bad LG, I is the best, and Cl & Br are good
what makes a good nucleophile in a substitution reaction
is measured in its reactivitiy
consider how strong the negative charge (the stronger, the more it wants to give it away and attack the Nu)
consider how EN (the less EN the weaker, as it means it is less stable with a negative charge, as it has less ability to pull electrons close to it)
consider the strength (pKa) of the conjugate acid (the weaker, the better Nu, as it means it is more basic in solution, more reactive to accept a proton = more likely to attack the positive EP)
if it has LP, consider how easy they are to protonate (easier to protonate, stronger Nu, as it means it is more reactive so will readily attack the positive on EP)
consider the strength of the bond being formed vs the bond being broken in LG (a stronger bond being formed makes the reaction exothermic, providing a thermodynamic driving force)
define an Sn2 reaction
Sn2 = bimolecular nucleophilic substitution
a way of characterising nucleophilic substituion reactions, to describe their rate
for when reaction rate is dependent on the concentration of BOTH reactants (BI molecular)
therefore reactants react with eachother directly, one the Nu and one the EP, in one reaction step (an elementary reaction)
this means there is one transition state, and no intermediate formed (transition state directly leads to the product)
describe an Sn2 reaction mechanism
occurs in one step, as rate is determined by conc of both reactants (EP & Nu, who directly react to form the product)
the negative charge of the Nu reactant, attacks the positive EP - transferring electrons to form a bond (e.g. C with a polarised bond from X), from the opposite side of the LG (both are electron rich and large, resulting in repulsion)
this forms the transiton state, where the Nu bond is starting to form with EP, and the LG - EP bond is starting to break (180 degree angle between Nu & LG, both with negative dipoles)
electrons are then transferred from the EP-LG bond, to the LG, and it leaves the molecule
this results in the substitued product
what would an Sn2 energy coordinate diagram look like
reactants will have higher energy than the products, as the reaction is exothermic due to stronger bonds formed in the products vs broken in the reactant
it has one peak, this being the transition state (highest energy, it must immediately go downhill - so downhill both ways in energy)
this represents it only having one step, so this must be the RDS - the energy of this peak vs the reactants being the activation energy
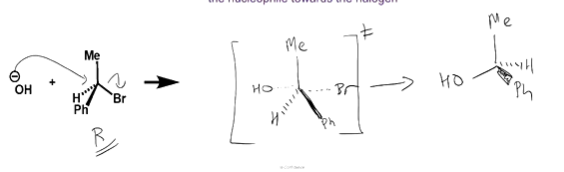
what is the stereochemistry of the product of an Sn2 reaction, and why?
the R groups are pushed from one side of the C, to the other, as the Nu attacks the C from the opposite side to the LG-C bond
an ‘umbrella inversion’ - substituents pushed inside out
so if starting as one enantiomer, the products will be the other enantiomer (e.g. R→S)
in the transition state, the stereochemistry of the R groups are the same as initially, while the Nu and LG are partially bonded / part way between bonding / breaking apart - the negative charge spread over them
what factors affect Sn2 reaction rate / likelihood of reaction
reaction rate determined by ability of Nu to get to EP (C) to begin reaction
mainly affected by EP carbon substituents
if big enough, may hinder Nu approach, and raise the energy of the transition state (harder to get to) - reducing rate
if anions, reduces the partial positive charge of C EP, so Nu is attracted less (reduces rate)
the more substituents (groups other than H), the more electron density, so Nu approach is more hindered (reducing rate) - so unsubstitued > primary > secondary > tertiary
for substitutions with EP & Nu that have slow reaction rates for either Sn2 or Sn1 reactions, what reaction would be likelier to occur?
likelier to do the reaction with a faster rate - so if hindered for Sn1 or Sn2, likelier to do the other reaction
define an Sn1 reaction
Sn1 = unimolecular nucleophilic substitution
a way of characterising substitution reactions which have multiple elementary steps - thus concentration of only one of the reactants determines the rate (the one undergoing the RDS)
the RDS involves only the EP forming an intermediate (used up in reaction), which then goes on to act as an EP, be attacked by the Nu, to form the product
Sn1 reactions often occur for highly substituted EP, which would hinder the Nu approach so much so that a Sn2 reaction would be very slow - therefore the additional reaction step makes the EP more available
describe an Sn1 reaction mechanism
the EP begins as a neutral molecule, the LG leaves (electrons in the bond not shared equally as the LG is more EN - forming a dipole and taking these electrons off the bond to leave) - this is the RDS
this is initiated by fragmentation of the molecule (e.g. instability due to C-LG bond dipole so LG leaves) - then with Nu present, it can attack
this forms a carbocation intermediate - C with only 3 groups & 6 valence e (empty P orbital)
the Nu then attacks the positive carbocation, and forms a bond as they share electron density - and substitution has occurred
this intermediate is high energy, so this step is quick as it wants to gain stability by releasing this energy, and the LG removal makes it easier for Nu to approach and provides it with more positive charge to attract
describe an Sn1 energy coordinate diagram
again, reactants have higher energy than products as this is an exothermic reaction where the bonds formed in the products are stronger than the bonds broken in the reactants
there are two peaks, each representing a transition state, which quickly form either the intermediate or the product, as they are highly unstable (break the rules of valence, cannot be isolated) - would be the partial bond of LG pulled away, and the partial bond of Nu joining EP
the dip of energy in between these two transition states is the intermediate (carbocation)
the activation energy is the change in energy between the reactant and the first transition state, this is the RDS as it requires higher energy than to go from the intermediate to the second transition state to form the product (the second activation energy)
what affects Sn1 reaction rate / likelihood of reaction
the more stabilised the carbocation, the easier / likelier it is to form, due to less energy being required to react form this transition state
it is stabilised by substituents (provide electron density to the positive charge, and gain a more stable steric arrangement with less repulsion from other groups) - therefore the higher degree of substitution on the C EP, the faster / likelier Sn1 will occur - tertiary > secondary > primary > unsubstituted
also consider LG ability (must be able to exist as stable anions, and be bonded weakly to the C - same as before)
this is because this step is required for the rest of the reaction to occur, for fragmentation of the molecule so Nu can attack
thus less dependent on Nu strengh (only dependent on LG ability)
describe the stereochemistry of an Sn1 reaction product
the intermediate carbocation involves 3 groups on a central C (6 total valence e all in bonding), thus the shape changes from tetrahedral → trigonal planar
this means when Nu attacks, it has an equal chance of attacking from either side of the planar (no LG attached that it must go opposite to like in Sn2) - above or below the plane
so the product forms a 1:1 racemic mixture of two enantiomers, each resulting from one direction of Nu attack, which when bonding pushes the R groups to the other side of the molecule
one enantiomer is the inversion of the reactant (opposite configuration / enantiomer to the reactant) - the other is the retention (same configuration / enantiomer to the reactant)
why is -OH a poor LG
how can it become better
-OH as an LG anion would be OH-, whose conjugate acid is H2O which is a weak acid, therefore OH- is a strong base - and a poor LG (unstable anion in solution so is unlikely to leave as molecules react to GAIN stability, rather than decrease stability in solution)
if we put this in acidic conditions, it becomes protonated (lone pair acts as a proton acceptor) to form -OH2+
this would make the LG H2O, which has a conjugate acid of H3O+ (strong acid), therefore H2O is a weak base - and thus a stable LG in solution, so likely to form
this may be Sn1 or Sn2 depending on the degree of substitution
describe an addition reaction
when two molecules are added together to form one molecule
one molecule with a pi (double bond) and one with a sigma (single bond), which upon combination, the pi bond is lost and 2 sigma bonds are formed in the product
what type of bond is a double bond
describe this
how is this different to the single bond
a double bond is made up of a pi bond and a sigma bond, rather than 2 single bonds (sigma bonds) - giving it unique chemistry
single (Sigma) bonds exist as an electron pair shared directly between two atoms in the bond
pi bonds instead exist as a cloud of electron density above and below the plane of the carbon atoms, shared between them
so a double bond has a sigma bond of a shared electron pair between carbon atoms, and a pi bond of electron density above and below the plane
the pi bond electron density is not held directly between the C, so is more available for reaction (Easier to break this bond, more reactive) - so this is the part (these electrons) that reacts while the sigma bond remains (more thermodynamic driving force to retain these)
so the energy of a double bond is not simply 2 sigma bonds added up, pi bonds are still strong, but not as strong as a sigma bond
what makes an addition reaction more reactive / likely to occur
alkene reactant stability
if have larger groups in closer proximity, there is more repulsion and steric strain, so less stability (more reactive and likelihood to react) - e.g. Z alkenes (trans isomers)
if more substituted, are more stable (less reactive, less likely to react), as electron density from the double bond is shared among more atoms to make the C more stable
describe the reaction mechanism of additon
(for symmetrical alkene x assymetrical reagent)
still use ideas of Nu attacks EP, the alkene acts as Nu (pi bond excess electron density), while the reagent acts as EP
the pi bond attacks the EP (e.g. proton, H, on H-Br) causing electrons to be pushed to the LG (so it can leave) while the pi bond attack then causes the EP to be bonded to the C in place of the double bond
this forms a carbocation intermediate, as the other C of the double bond has now lost electrons (have been moved to the bond with EP, H), so it has a + charge - becoming an EP
the reagent LG (now a Nu, has gained electrons) then attacks the carbocation EP, transferring electrons to then form another single bond
this generates our product, with 2 groups from the reagent, joined onto the C where the double bond was
describe the reaction coordinate diagram for addition
(symmetrical alkene x assymmetrical reagent)
reactants have higher energy than the products, as the reactants are exothermic, due to bonds formed in the product being stronger than the ones broken in the reactants
two peaks, the first one representing the transition state as the C of the alkene is protonated (pi bond partially broken, proton partially bonded to alkene while still partially bonded to the LG)
this is the RDS, requires the most energy, so provides us Ea
it then dips, representing the intermediate (Carbocation)
then it goes to the second peak, the next step where the reagent LG Nu attacks the carbocation EP, which requires less energy as the carbocation is high energy
after it passes this transition state, the product is formed
what is the Markovnicov rule
this occurs in alkene addition with assymetrical reagents, where there are potential isomers formed in the product (multiple products that can be formed), as their are options as to what part of the reagent will go on what part of the alkene
but there is usually one major isomer, all product formed as this, or mostly (and only some of the other isomer)
‘rich gets richer’ - in addition as the double bond is broken, the C with the most substituents will gain another substituent, and the H will go on the other C - most often, forming this as the major product
in other words, the more electronegative component of the reagent, acts as this subtituent to add to the more substituted C, while the other part (e.g. H) adds to the least substituted C
how do addition reaction mechanisms explain Markovnicov’s rule
this is explained by the mechanism, as with a symmetrical alkene + assymetrical reagent, there are two possible places for the alkene to react / form this carbocation / allow each group to join
the one more likely to occur, will be the one that forms the most stable carbocation (more substituted, more C-R bonds to provide electron density for the C to pull towards itself (more so in C-C than C-H) to minimise this + cation charge)
this is because, it requires the least energy to go through the transition state to make this carbocation, so it will be favored in reaction and form more (tertiary > secondary > primary)
what is anti-markovnicov addition
how do we make this occur
addition forming the minor product, the product that doesnt follow markovnicov’s rule (C with the most substituents gains a susbtituent / most EN part of the atom, C with the most H gains another H)
doesnt happen naturally (or only in small amonts), so requires different reagents - not chemically favored as it requires more energy to form the carbocation intermediate, for the reaction to occur
(i) B2H6
(ii) H2O2, NaOH
how do different assymmetrical addition reaction reagents do the same overall thing?
what are two types of FG interconversions that involve this?
H-X (alkene → hydroalkane)
simplest, e.g. H-Br, H-Cl
these just provide H as the EP which is attacked by alkene double bond Nu to join first, then the X LG gains electrons, exists as an anion in solution, then comes back to act as Nu to join onto the carbocation
H2SO4 & H2O (alkene → alcohol)
H2SO4 does the first part of the reaction, as it is an acid it acts as an H+ donor, so its proton is attacked by the alkene double bond Nu to join onto the molecule first, to form the carbocation
the H2O then does the second part of the reaction, its lone pairs on O act as Nu to attack EP carbocation, and join as an H-O+-H (protonated hydroxyl group)
this protonated group is then deprotonated, as the electrons in one bond with H are transferred to the + charged O (forming -OH group)
describe the reaction mechanism for additon with a symmetrical reagent
provide an example, and name the FG interconversion that involves this
must consider why does one part of the reagent go on one C of the double bond and vice versa
(alkene → dihaloalkane)
e.g. Br2, Cl2, I2,
initially alike addition with assymmetrical reagents,
alkene pi bond acts as Nu, while an X of the X-X acts as EP, as this bond is highly polarisable, and recognised as an atom bonded to an EN atom (even though they are the same) - this attacks and joins one X to the molecule, as the other X is the LG so becomes an anion
this then forms a carbocation, but the one joined X has lone pairs to act as Nu and attack the positive charge, forming a bromonium cation as the one Br group is bonded to both C of the double bond, itself with a + charge (internal nucleophilic substitution) - as Br is very large (many electron shells) - this occurs directly as the intermediate (no carbocation intermediate)
the leftover X- LG then acts as Nu to attack the C-X bond (slight positive as X pulls electron density from C), resulting in electrons transferred from the bond to the positive Br
therefore the initial Br is bound to one C of the double bond, then the LG Br is bond to the other C of the double bond (on the other side due to stereochemistr)
describe the stereochemical outcome of symmetrical reagent alkene addition
this is determined by the formation of the bromonium cation intermediate
the first joining of X involves the pi bond electron cloud (above or below the plane), so the first X sits on top of the alkene
but the second X joining (as a Nu LG) comes in from the opposite side (due to the X being large and the LG being large), so it joins to the opposite side of the initial X
this forms a trans product in cyclic molecules
what may change the process of symmetrical reagent alkene addition, to result in a product that does NOT have two identical groups (of the original symmetrical reagent)
we may have the initial X-X symmetrical reagent doing the first step to form a bromonium cation
but then depending on the concentration of the X- LG generated, vs other anions in solution, we may have another anion in solution attacking the molecule instead of the original X- LG
competing Nu may be water, NaCl’s Cl-, or any other Nu that is better and in higher conc.
e.g. Br2 & NaCl, Br2 attacks initially to form bromonium cation, then Cl- from NaCl is in higher concentration so attacks this to form 1 x Br on the molecule and 1 x Cl, a dihaloalkane but not with two identical halogens
how does alkene addition with a symmetrical reagent work with cycloalkenes
just the same way as regular alkene addition
the first X EP being attacked by the Nu electron cloud on top of the molecule, then the other X Nu attacking from opposite to the first joined X, forms a trans product
therefore one X is above the plane of the ring, the other below the plane, for the two Cs originally in the double bond
describe hydroxylation
what type of reaction is this
what FG can it convert between , using which reagents
an addition reaction, joining H & OH (hydroxyl) to the molecule
mechanism is beyond this course
forms an alcohol from an alkene (hydroxylation), with a major and minor product
for the major product use H2SO4 / H2O
for the minor product (antimarkovnicov addition) use (i) B2H6 (ii) H2O2, NaOH
what is hydrogenation
what type of reaction is this
what FG can it convert between, using which reagents
addition involving adding H2 to the molecule, to form an alkane from an alkene
mechanism is beyond this course
converts alkene → alkane by adding an H to each C of the double bond, with H2 reagent & Pd catalyst (catalytic hydrogenation)
describe an elimination reaction
name the main type of one
what FG does it convert between, using which reagent
the opposite of an addition reaction, removing something from the molecule and forming a double bond in its place
not looked at in detail for this course
(dehydration) removal of water (H & OH) to create an alkene
does alcohol → alkene, by removing H & OH to form water, and forming a double bond in its place (excess H3PO4 & heat)
this can be used to change the position of -OH alcohol FG, by forming an alkene then doing antimarkovnicov addition to form the other product (1: B2H6 2: H2O2, NaOH)
what is an aromatic molecule
give the most common example
molecules with a structure alike benzene - they have a 6-membered ring, with arangement of electrons in pi bonds - can involve other atoms and FG, and be multiple rings fused
the 6 pi electrons are delocalised, above and below the 6C ring, so the base ring looks like each C having a cloud above and below of these pi electrons
these pi bonds are a cycle of 3 in a continuous ring of swapping around the C atoms (resonating with 2 degenerate resonance contributors - have the same energy)
what is the consequence in terms of stability for aromatic molecules
they are extra stable due to their resonance, not just pi bond electrons on one molecule, they are shared among a ring constantly interchanging
this makes benzene and other aromatic molecules less reactive
how do you name aromatic molecules
many have common names which are used instead of their technical names based off benzene
for multiply substituted benzene rings, can use numbers / words as prefixes to the common name / original name / benzene, based on the site of theses substituents
(ortho / o) = substituent on the C next to the C with the first substituent
(meta / m) = substituent on the 3rd C of the ring (second away from the first substituent)
(para / p) = substituent on C4, parallel to the first subsitituent
e.g. m- methyl nitrobenzene (common name = m-nitrotoluene)
e.g. o - dihydroxybenzene (without o is 1,2 dihydroxybenzene)