N5 1.1 Rates of reaction
1/20
Earn XP
Description and Tags
Flashcards covering key concepts related to chemical reaction rates, factors affecting them, and measurement techniques.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|

21 Terms
Reaction rate
Describes how quickly products are formed during a chemical reaction.
End-point
The point in time when the reaction is complete (no more products formed).
Catalyst
A substance that speeds up chemical reactions but can be recovered chemically unchanged at the end of the reaction.
Effect of decreasing particle size on reaction rate
Increases the reaction rate.
Effect of increasing concentration on reaction rate
Increases the reaction rate.
Effect of increasing temperature on reaction rate
Increases the reaction rate.
Method to collect a soluble gas
Gas syringe.
Reason for reaction rate decrease as reaction proceeds
The reactants are getting used up.
During a reaction, the mass of gas produced was measured in g. Time was measured in s.
Average rate will have units of …
g s-1
During a reaction, the volume of gas produced was measured in cm3. Time was measured in min. Average rate will have units of …
cm3 min-1
If a reactant is ___________, adding more of it will not make more products.
in excess
Method to collect an insoluble gas
Inverted measuring cylinder or gas syringe.
Method to monitor reaction rate without collecting a gas
Measuring mass loss.
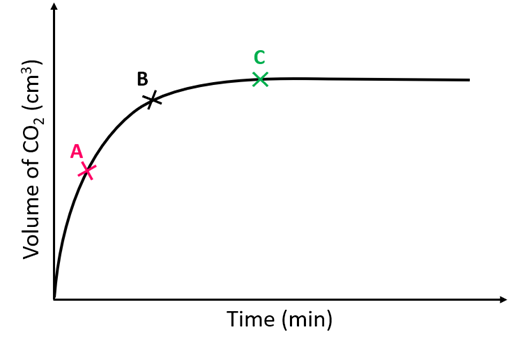
At which point (A. B or C) is the reaction complete?
C
Reaction rate can be increased by decreasing the…
particle size
Reaction rate can be increased by adding a …
catalyst
State the formula used to calculate average rate. Where can you find it?
rate = change in quantity / change in time
Can be found in the data booklet
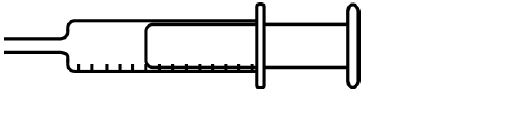
Draw a diagram of a gas syringe
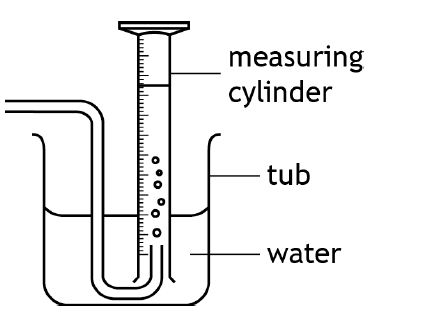
Draw a diagram of collecting a gas over water.
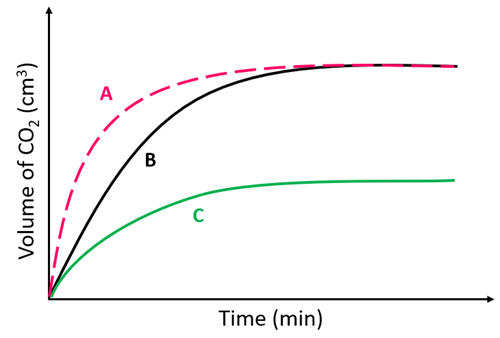
A reaction between zinc powder and sulfuric acid produced graph A.
Which graph will be produced if zinc lumps are used?
B
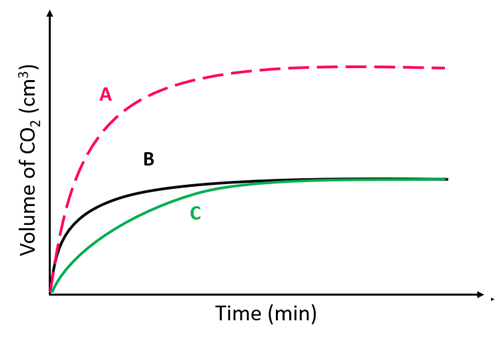
Reaction C was carried out using an excess of zinc and 1M sulfuric acid.
Which curve shows the reaction progress when 2M sulfuric acid was used?
A