MASTER MCAT PHYSICS
1/139
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
140 Terms
c = 3 × 10^8 m/s
speed of light in a vacuum (c) = ?
directly related
intensity (and energy) of light is _______________ (in/directly) related to the amplitude of light
radio wave < microwave < IR < visible light (ROYGBV) < UV < x-rays < gamma rays
rank from least to most energy (and frequency)
visible light (ROYGBV), x-rays, UV, gamma ray, IR, microwave, radio
gamma rays < x-ray < UV < ROYGBV < IR < microwave < radio wave
opposite of frequency and energy
rank from shortest to longest wavelength:
visible light (ROYGBV), x-rays, UV, gamma ray, IR, microwave, radio
reflection
refraction
absorption (don’t worry about it)
2 things that can happen when light encounters an interface between 2 media
reflection
the act of light bouncing off a given medium
angle of incidence EQUALS angle of reflection
refraction
wave bends when entering a new medium (its speed and angle refraction change, BUT frequency stays constant)
angle of refraction
speed
what 2 factors change when light is being refracted?
index of refraction
tells the ratio of the velocity in a vacuum (c) in relation to the velocity the medium (v)
n = c/Velocitymedium
index of refraction formula (n)
n = c/Velocitymedium
Snell’s law (calculate refraction) formula
n1sinθ1 = n2sinθ2
n is found from index of refraction formula: n = c/Vmedium
θ is the angle of refraction
inversely
when n increase, angle refracted decrease, and vice versa
index of refraction (n) is ____________ (directly/inversely) related to angle refracted (θ2)
toward
bc velocity would DECREASE, so light would fall closer to the 90 degree line perpendicular to medium
if n2 > n1, light bends ____________ (toward/away) from the normal
away
bc light travels faster with a smaller index of refraction, so it’d deviate from the normal
if n2 < n1, light bends ____________ (toward/away) from the normal
total internal reflection
light transmitting to a medium with a LOWER refractive index (does NOT refract → no bending light, 100% reflected, 0% refracted)
2 conditions must be met:
n2 < n1 so sinθ2 > sinθ1
increasing θ1 leads to an even greater θ2
n2 < n1 so sinθ2 > sinθ1
ex: n1 is water or glass, and n2 is the air outside (n2<n1)
increasing θ1 leads to an even greater θ2
what are the two conditions of total internal reflection?
critical angle (θc)
the MINIMUM angle possible in order to maintain total internal reflection
in other words, it is θ1 at which θ2 = 90 degrees (max value bc that’s perpendicular to the NORMAL)… sinθc = n2/n1
90 degrees!
when θc is plugged into n1sinθc, then θ2 = ?
diffraction
dispersion
polarization
what are the 3 wave effects that light waves undergo?
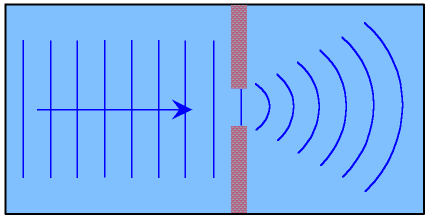
diffraction
when waves encounter a small slit that’s same size as its length or smaller, the waves spread out. this is observable by light/dark pattern on a screen
dispersion
when wave spreads out all over bc its speed depends ONLY on the medium, not on frequency (which is not true for light)
different colors have different indexes of refraction → bends differently
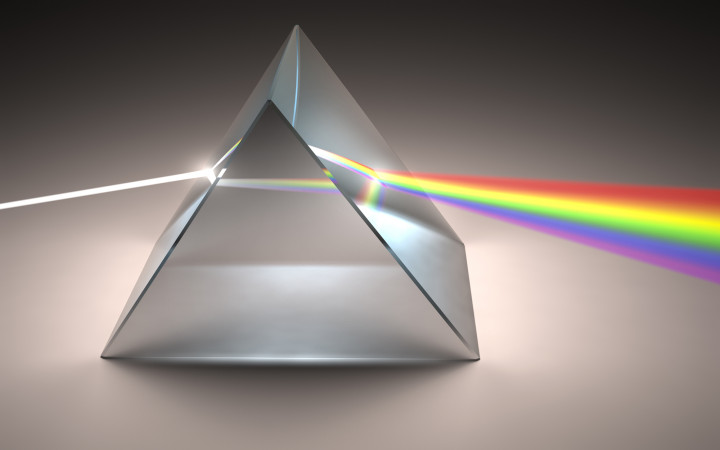
faster, more
short wavelength = more energy/frequency, so waves travel faster, which leads to more bending
in terms of light dispersion, the shorter the wavelength, the ____________ (slower/faster) light travels in glass/water —> ___________ (more/less) light bending
polarization
wave effect that occurs when 1 direction of oscillation is PREFERRED over the other, either by reflection/transmission through medium (for transverse waves ONLY, not sound)
focal length (f)
the intrinsic property of lens/mirror that depends on index of refraction and curvature of the lens/mirror
convex/converging lens
type of lens that gives a positive focal length. lens that allow refracted light to meet → makes a real image (+i)
concave/diverging lens
type of lens that gives a negative focal length. lens that does not allow refracted light to meet → makes a virtual image (-i)
magnification (m)
the size of the image compared to the object that image came from
what does a positive magnification (+m) mean?
upright image
what does a negative magnification (-m) mean?
inverted image
focal length (f) formula
1/f = 1/o + 1/i
+f for convex/converging lens
-f for concave/diverging lens
inverted, -m
all real images (+i) are __________________ (inverted/upright) with a ___ (±) m
upright, +m
all virtual images (-i) are __________________ (inverted/upright) with a ___ (±) m
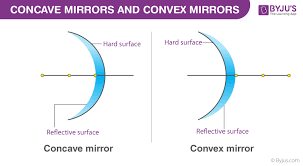
converge, positive f
concave mirrors _______________ (converge/diverge) light → f is __________ (positive/negative)
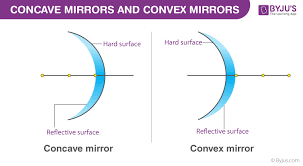
diverge, negative
convex mirrors _______________ (converge/diverge) light → f is __________ (positive/negative)
1/f = 1/i + 1/o m = -i/o
concave MIRROR → +f
1/6cm = 1/i + 1/15cm → i = 10cm
-10cm/15cm → m = -2/3.
image is inverted and 2/3 compared to size of object
object is placed 15 cm away from a concave mirror with focal length of 6cm. where is the image being formed? and how big is the image compared to the object?
magnification (m) formula
m = -i/o
myopia (near-sightedness)
condition where focal length of eye’s lens system is TOO SHORT. need glasses that’s a diverging lens to form image at retina.
diverging lens
people with nearsightedness (myopia) need __________ (converging/diverging) glasses lens
lens achieve clear vision by REFRACTION
squinting eyes achieve vision by DIFFRACTION
lens achieve clear vision by REFRACTION
squinting eyes achieve vision by DIFFRACTION
lens power
your glasses prescription (nearsighted = negative prescription… think about why. hint: what type of lens do nearsighted ppl use?)
P = 1/f
lens power formula
P = 1/f (in m^-1)
P = 1/f 1/f = 1/o + 1/i
-5 = 1/f → f = -1/5
-1/5 = 0 + 1/i (bc o was not given) → i = -1/5 = -0.2m
suppose your glasses have power of -5. where do your glasses form an image of a distant object?
hyperopia (far-sightedness)
condition where focal length of the lens’ system is TOO LONG. needs converging (convex) lens (positive prescription)
E = mc²
formula that relates mass to energy? (hint: albert einstein)
E = hf = hc/(lambda)
planck’s constant (h) = 6.62 × 10^-34
formula that relates nrg to frequency, plancks, and wavelength (2 formulas)
A.
convex lens produce real images bc the rays converge (meet), at which the image is inverted
what type of image is formed by a convex lens when the object is placed beyond the focal point?
A. real and inverted
B. real and upright
C. virtual and inverted
D. virtual and upright
A.
1/f = 1/i + 1/o m = -i/o
1/10 = 1/i + 1/15 → i = 30cm (pos bc concave mirror converge light)
m = -30/15 = -2. inverted and magnified
a concave mirror has a focal length of 10cm. an object is placed 15cm away from the mirror. what is the nature of the image formed?
A. real, inverted, and magnified
B. real, inverted, and reduced
C. virtual, upright, and magnified
D. virtual, upright, and reduced
B.
which of the following best describes the behavior of light rays when they pass through a converging lens?
A. light rays diverge after passing through the lens
B. light rays converge to a focal point after passing through the lens
C. light rays reflect off the lens in random directions
D. light rays continue in a straight line after passing through the lens
C.
they are asking to find lens power (P) = 1/f.
1/f = 1/o + 1/i
o = 1.0m, i = 0.02m
P = 1/f = 1/1 + 1/0.02
1/0.02 = 100/2 = 50 → 1 + 50 = 51 D
A person, whose eye has a lens-to-retina distance of 2.0 cm, can only clearly see objects that are closer than 1.0 m away. What is the strength S of the person’s eye lens? (Note: Use the thin lens formula .)
A. –50 D
B. –10 D
C. 51 D
D. 55 D
B.
remember ohm’s law: V = CR (voltage = current * resistance)
When the current in a machine’s circuit increases and the resistance of the circuit remains constant, the voltage of the circuit:
A. decreases
B. increases
C. is constant
D. is zero
B.
n = c/v (c = speed of light)
n = 3E8 / 2.1E8 ~ 1.5
Knowing that the speed of light in the vitreous humor is 2.1 × 108 m/s, what is the index of refraction of the vitreous humor? (Note: The speed of light in a vacuum is 3.0 × 108 m/s.)
A. 0.7
B. 1.4
C. 2.1
D. 3
heat (Q)
transfer of non-mechanical energy b/w a system and its environment
related to the total thermal nrg of an object (measured in joules)
temperature (T)
macroscopic measure of the average internal (thermal) nrg of a system
energy (E) is the TOTAL energy, but the temp (T) measures the energy (E) of the INDIVIDUAL molecule
how are energy and temperature different?
intensive property
property that does NOT depend on the amount of material
extensive property
property that depends on the mass of material
closed system
system that allows NO exchange of matter
molecules cannot go in and out of the system
BUT still allows work to be done
Q = mCΔT
m = mass
C = specific heat of molecule
ΔT = temp change
formula for heat (Q)
phase change
process where system changes phase (gas, liquid, solid)
naur <3
its phase can change (solid, liquid, gas)
does adding heat to the system always change its temperature?
T increases and/or gas expands (or both)
If Q > 0 for an ideal gas:
what happens to T (increase/decrease)?
gas (expands/constricts)?
zeroth law of thermodynamics
law that states when 2 substances are in contact, heat transfers b/w them until they achieve the same temperature (thermal EQ)
conduction
convection
radiation
what are the 3 modes of heat transfer?
conduction
heat transfer through solids in contact
conduction
when you place a metal spoon in a hot cup of tea or coffee. The heat from the hot liquid is conducted through the metal spoon and into your hand as you hold it, warming your hand.
which heat transfer method is this an example of?
convection
heat transfer through fluids circulation
convection
when you boil water in a pot on a stovetop. As the water is heated, it becomes less dense and rises, carrying heat energy with it. Cooler water then moves in to take its place, and the process repeats, creating a continuous cycle of heat transfer through the movement of the water.
which heat transfer method is this an example of?
radiation
heat transfer by emission of electromagnetic energy (EM nrg)
radiation
the warmth you feel from the sun on a sunny day.
which heat transfer method is this an example of?
ΔE = Q - W
Q = heat added to the system
Q > 0: heat ADDED to system
Q < 0: heat LEAVES the system
W = work done BY the system
W > 0: when system DOES work
W < 0: when work done ON system
what is the internal energy of a closed system? (ΔE)
heat gets added to the system
what happens to the internal energy when Q > 0?
system does the work
what happens when W > 0?
work is done ON the system
what happens when W < 0?
system DOING work
W > 0
is expansion an example of work done ON the system or the system DOING the work?
also does that mean W __ 0? (<,>)?
work done ON the system
W < 0
is contraction an example of work done ON the system or the system DOING the work?
also does that mean W __ 0? (<,>)?
PV = nRT
ideal gas law formula
work (W)
gas expands → V increases → W > 0
gas contracts → V decreases → W < 0
for ideal gas, volume (V) is related to ___________
V increases, W > 0 (system does work)
when gas expands, what happens to volume (V) and work (W)?
V decreases, W < 0 (work done ON system)
when gas contracts, what happens to volume (V) and work (W)?
energy (E)
for ideal gas, temperature (T) is related to ___________
Q ≠ 0, W ≠ 0, or both
but E = Q - W still though
if an ideal gas transitions from 1 state to another (gas, solids, liquids). what happens to Q and W?
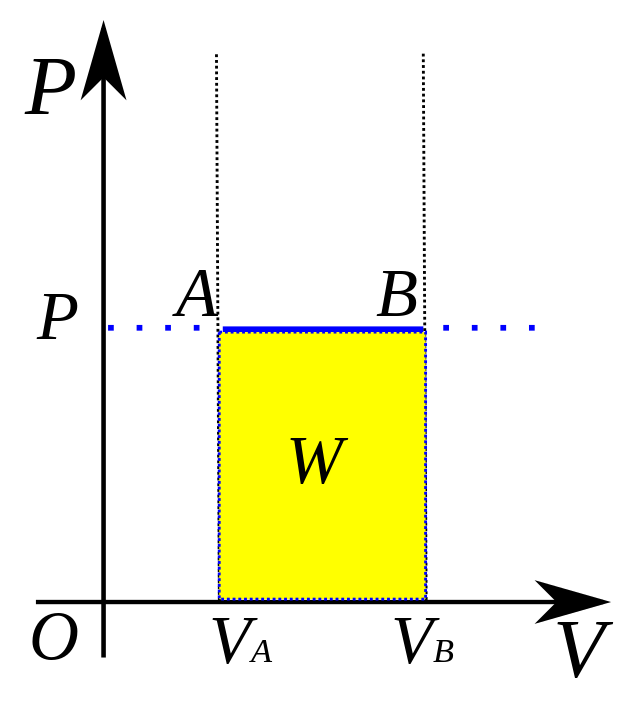
isobaric process
P = force / area
system has a constant pressure (ΔP = 0)
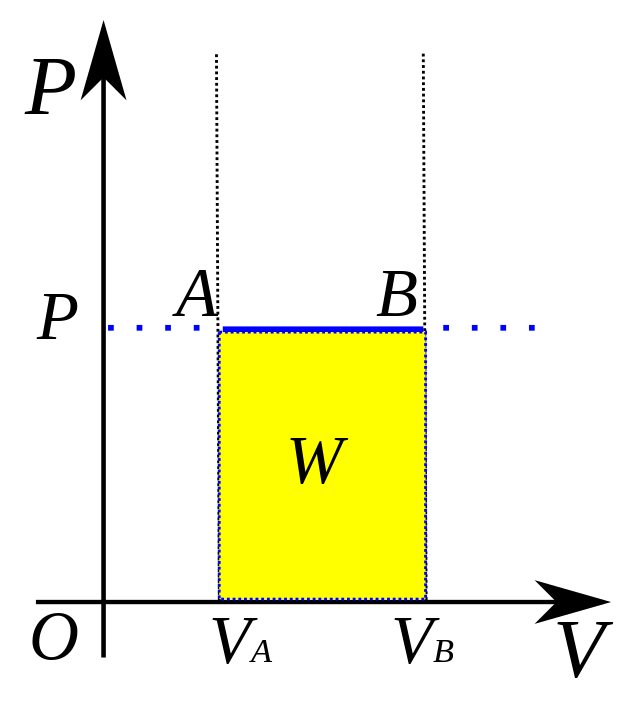
P = force / area
formula for isobaric processes to find pressure?
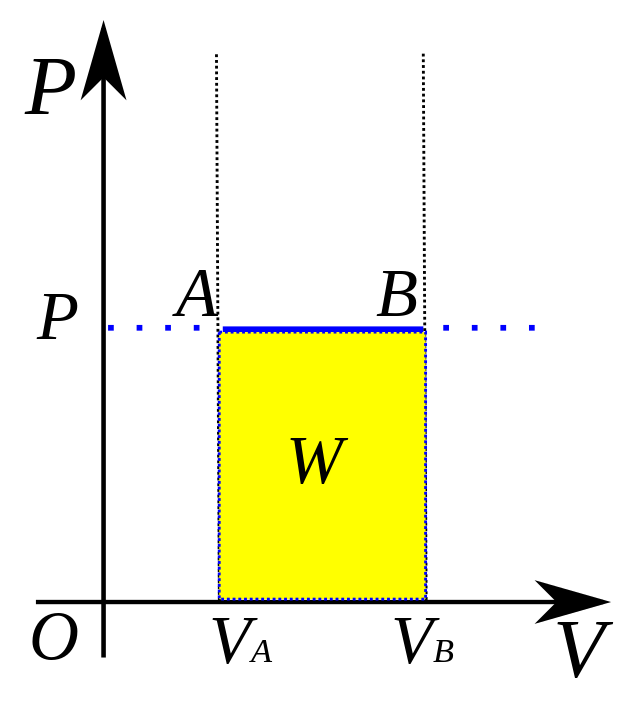
W = PΔV
SO… ΔE = Q - PΔV
formula to find work (W) and energy change (ΔE) in an isobaric system (ΔP constant)
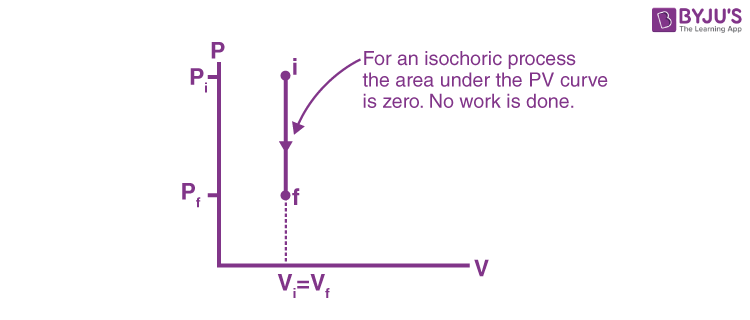
isochoric process
system has a constant volume (ΔV = 0)
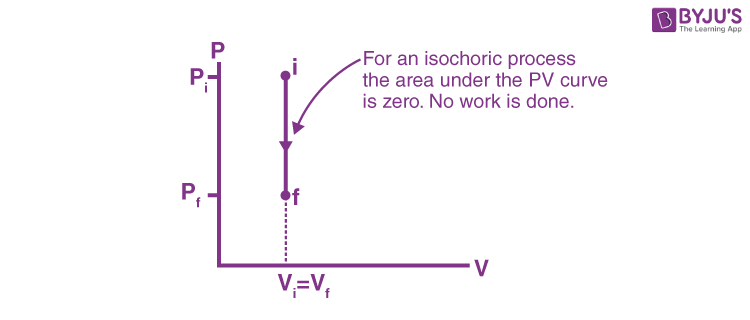
temperature rises… so pressure rises, and that’s it
for isochoric processes, what happens when heat is added? (Q > 0)
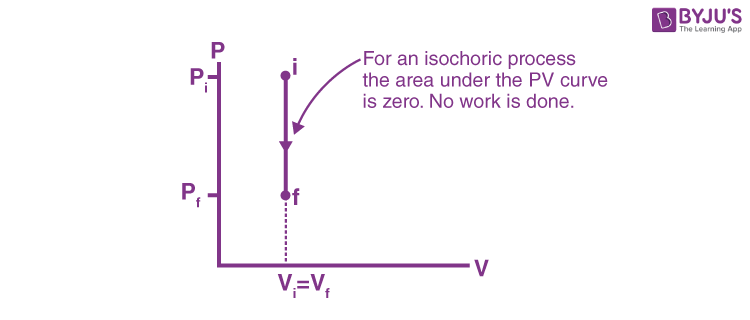
ΔE = Q (no W)
formula to find energy change (ΔE) in an isochoric process?
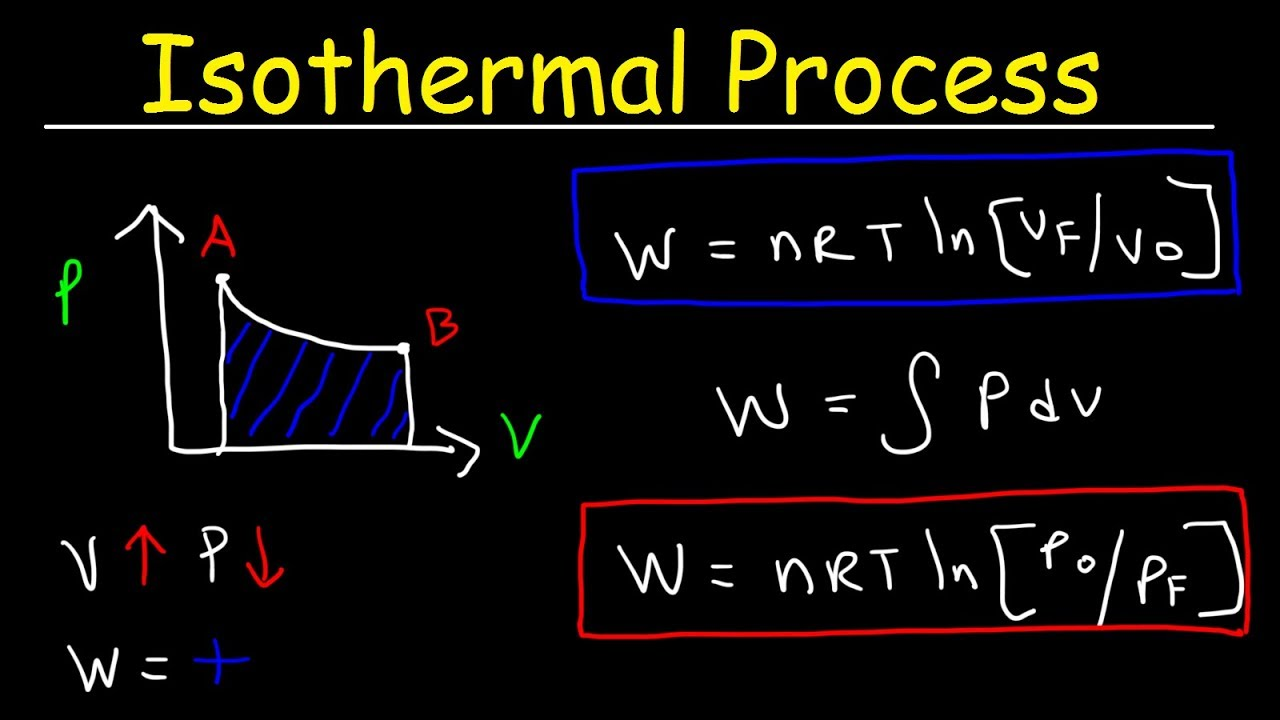
isothermal process
system in which temperature stays constant
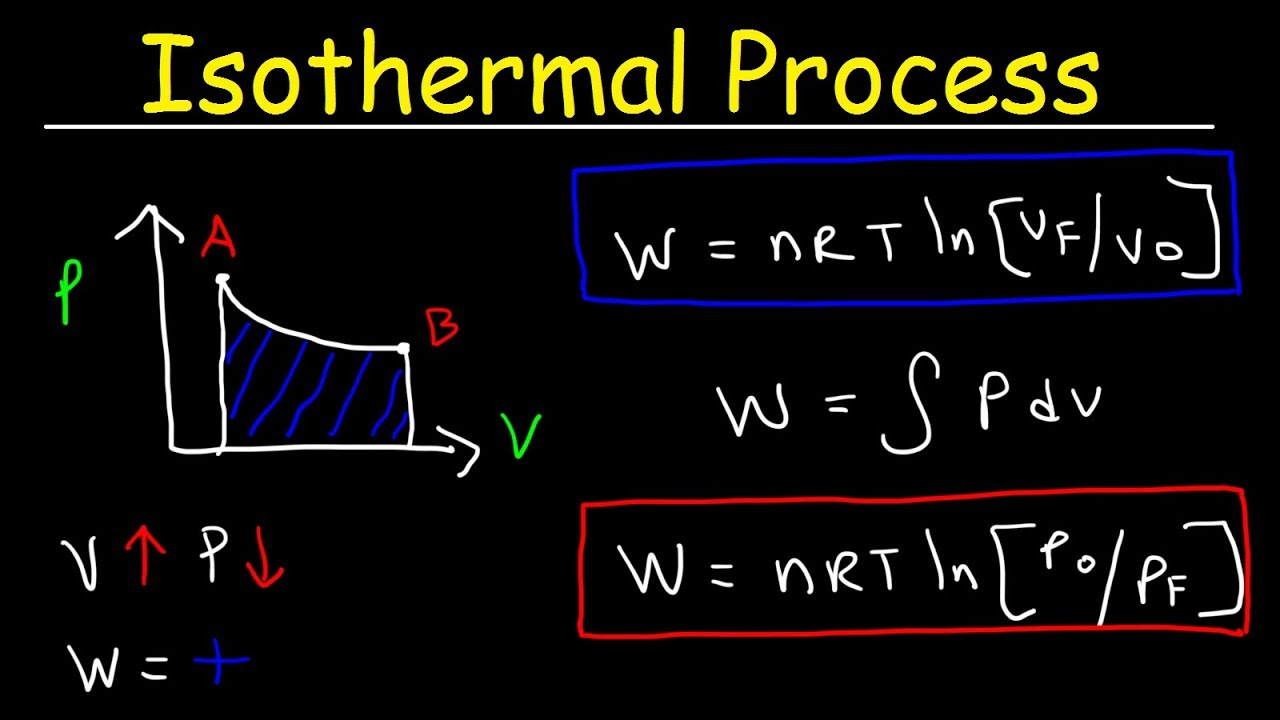
volume increase… so pressure decreases
what happens to isothermal process when heat is added? (Q > 0)
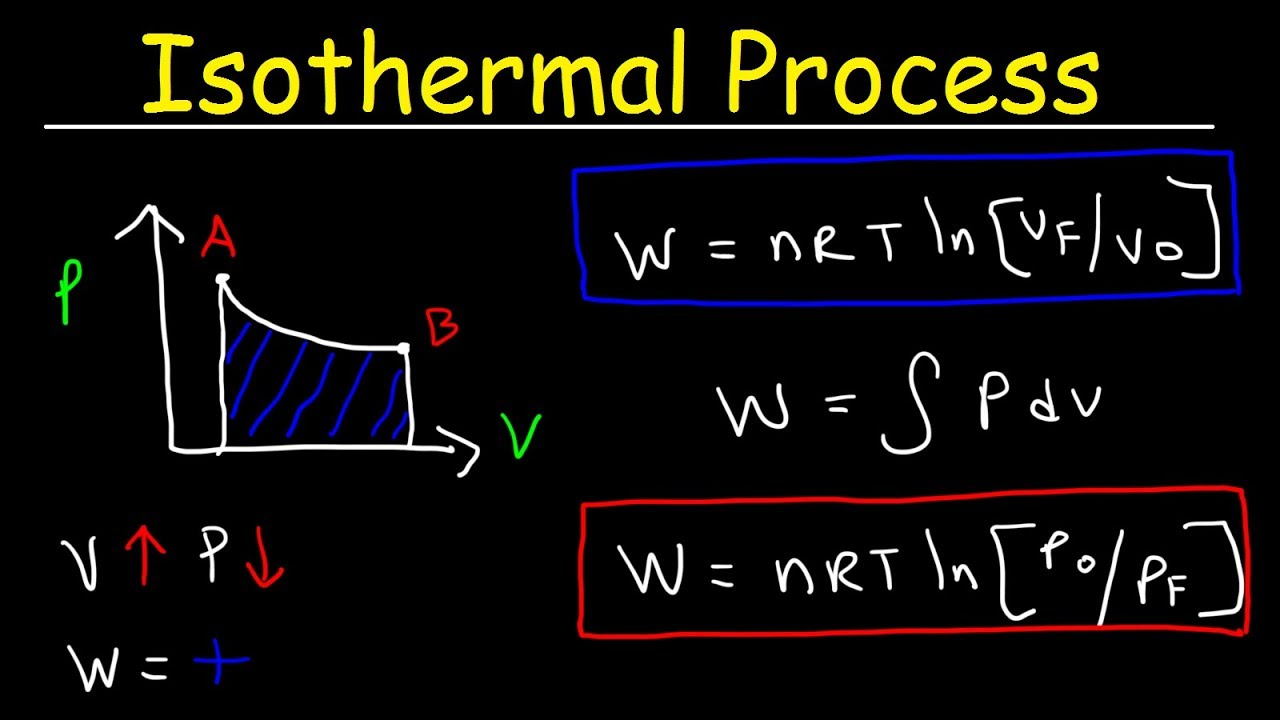
Q = W
since temp change is 0, ΔE is 0 bestie
formula to find energy change (ΔE) in an isothermal process
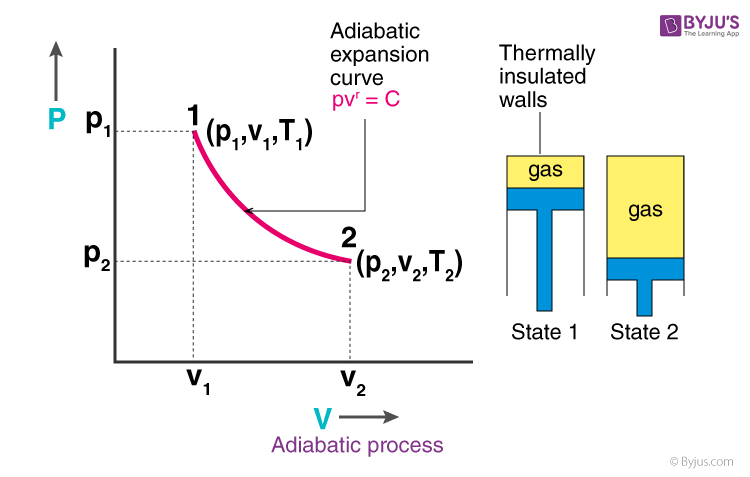
adiabatic process
system in which there is no heat transfer (ΔQ = 0)
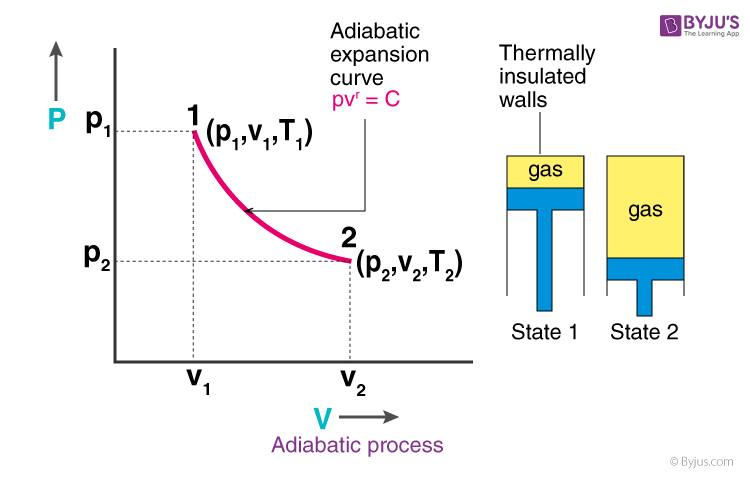
volume increases, pressure decreases, temperature decreases → nrg decreases
temp decrease bc gas molecules are doing work on the surroundings as they expand → reduction in kinetic energy
if volume increases in an adiabatic system, how does it affect V, P, T, and E?
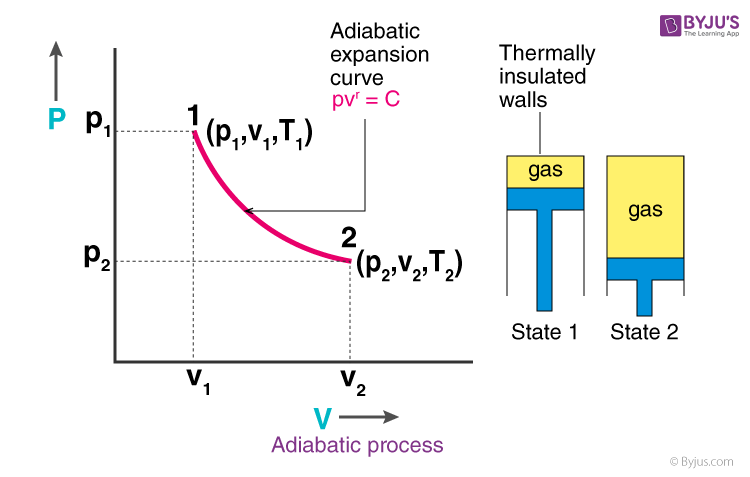
temp decrease bc gas molecules are doing work on the surroundings as they expand → reduction in kinetic energy
why does temp decrease and energy decrease in an adiabatic process when volume increases?
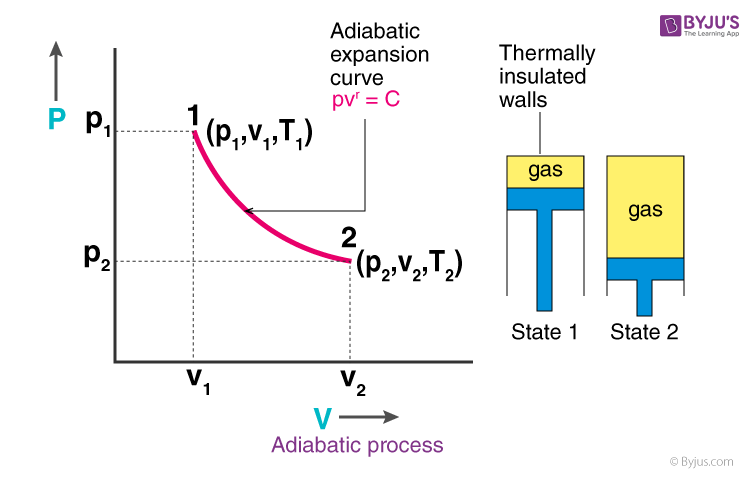
ΔE = -W
formula to find ΔE energy change in an adiabatic system
entropy
measure of disorder of the system
isolated system
system in which neither energy nor matter is exchanged with the outside
(ΔE = Q - W cannot be applied)
closed system
system in which matter is NOT exchanged with outside environment, but energy can come in and out (ΔE = Q - W)
either stays the same or increases during any thermodynamic processes
how is entropy in an isolated system look?
can decrease, but only if the entropy of its surrounding environment increased by a greater amount
can entropy of a closed system decrease?
density = mass / volume
density formula