Cell Bio - Exam 3
1/261
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
262 Terms
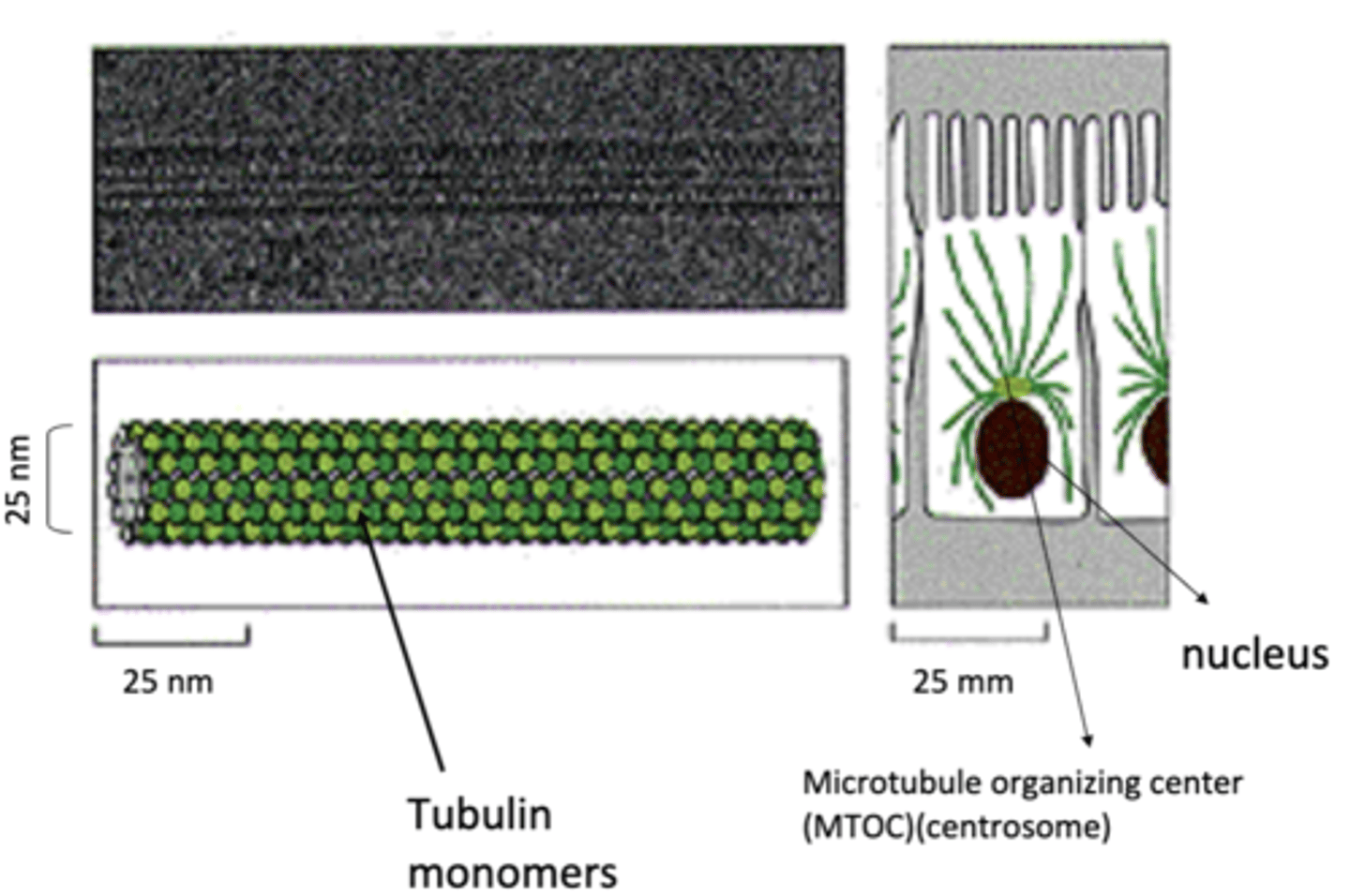
25 nm
diameter of microtubules
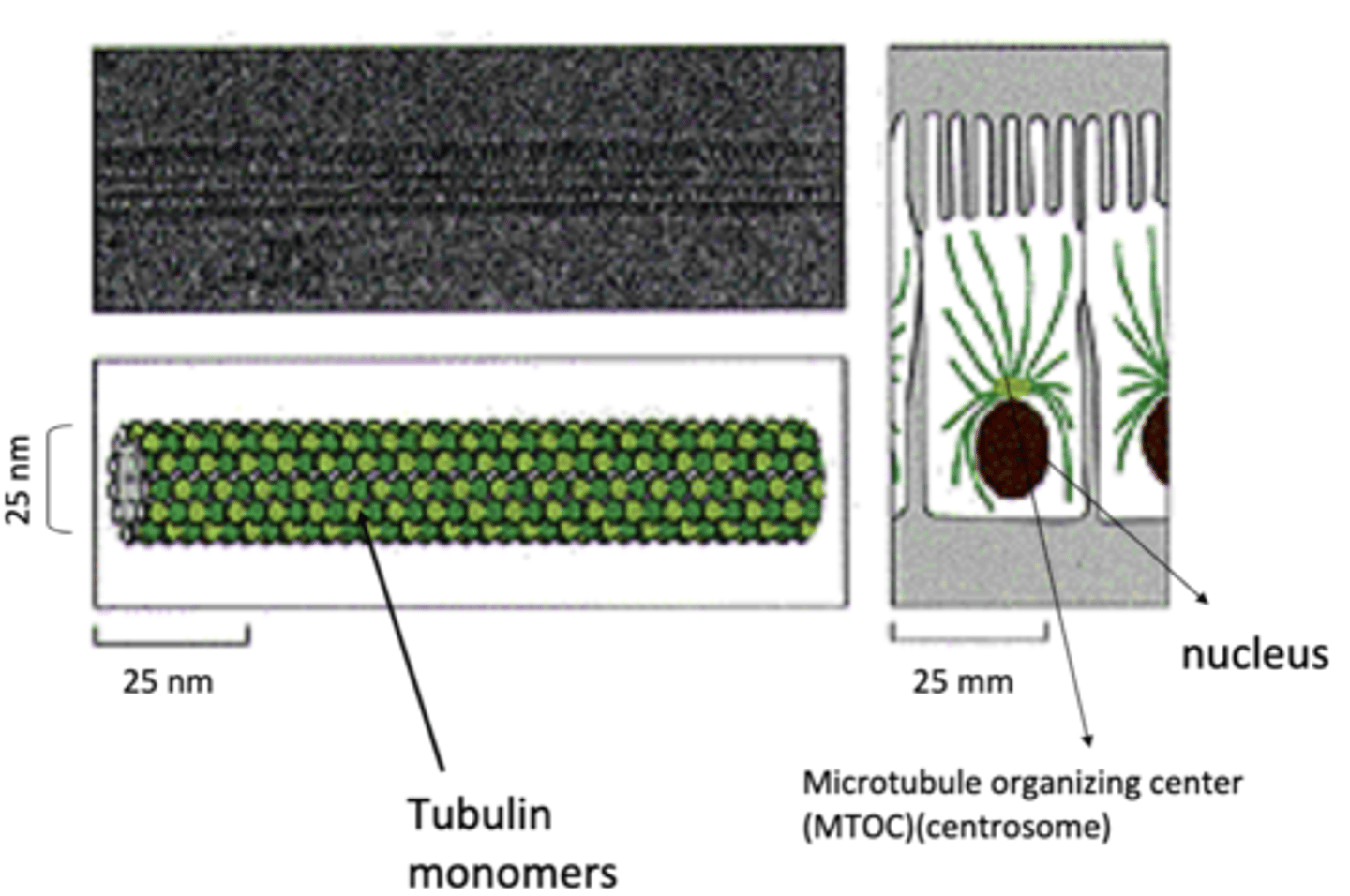
microtubule organizing center (MTOC)
most microtubules emanate from a ______________ ______________ _____________ (____)
tubulin
microtubules are made of _______________ monomers

1. organization of intracellular organelles and transport of vesicles (motor proteins)
2. beating of cilia and flagella
3. structural integrity of nerve cell, red blood cell, and flagellar structure
4. alignment and separation of chromosomes during mitosis and meiosis
microtubules play a role in
heterodimers, α-tubulin and β-tubulin
microtubules are made of _____________dimers that consists of a _______-__________ and _______-__________
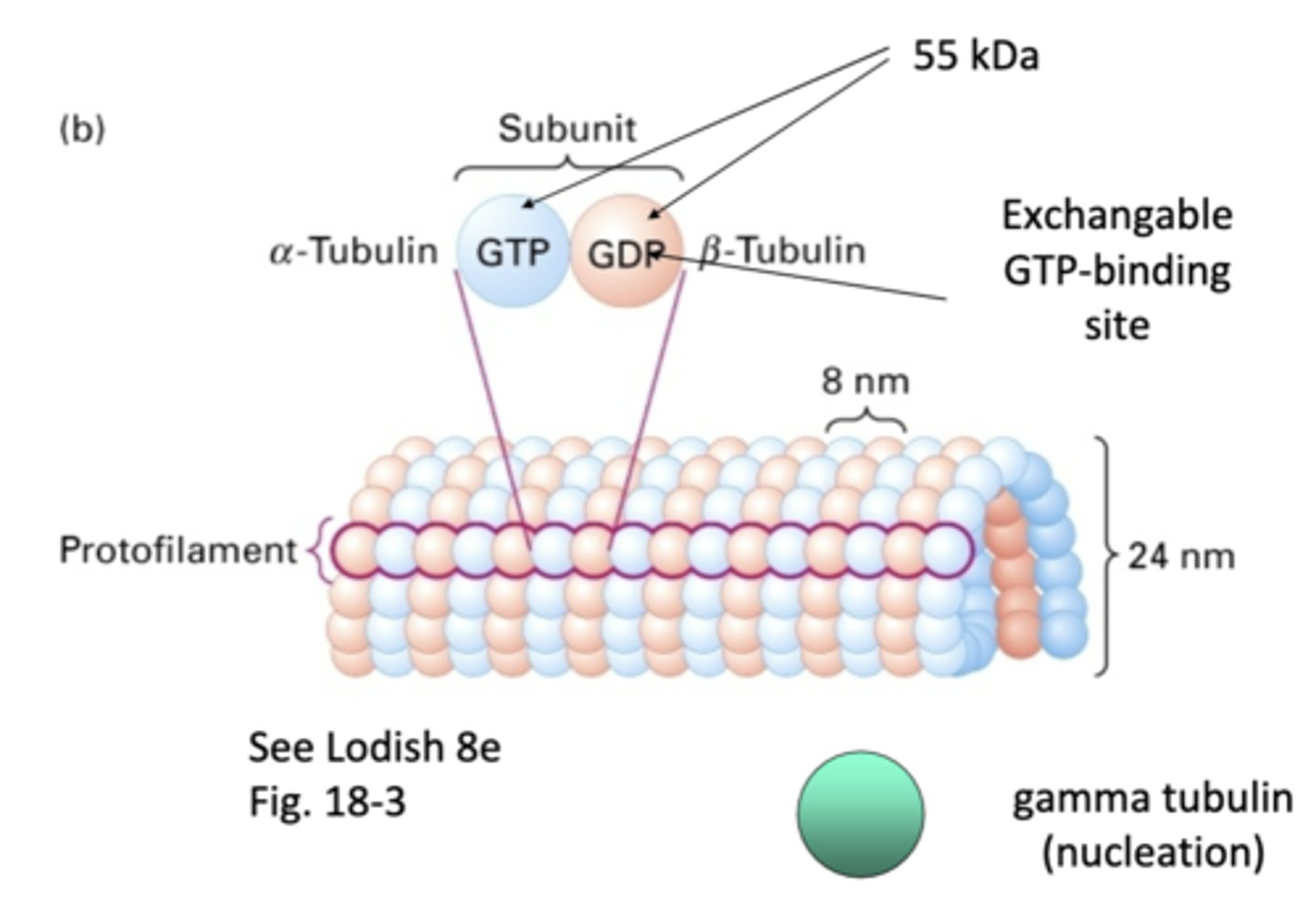
1 α-tubulin and 1 β-tubulin
one subunit of a microtubule consists of
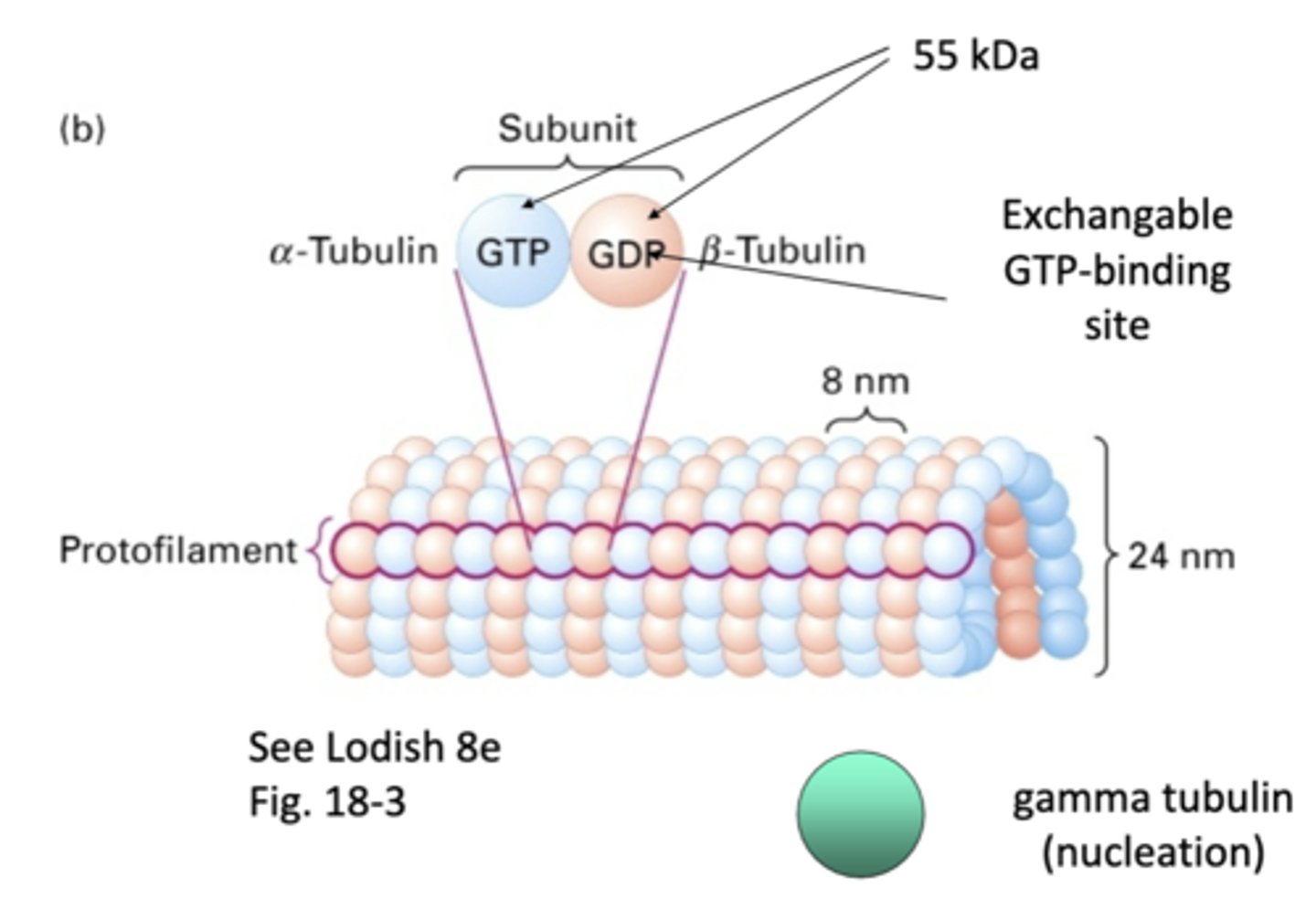
α-tubulin, β-tubulin
_-tubulin has permanent GTP while _-tubulin is GDP/GTP exchanged
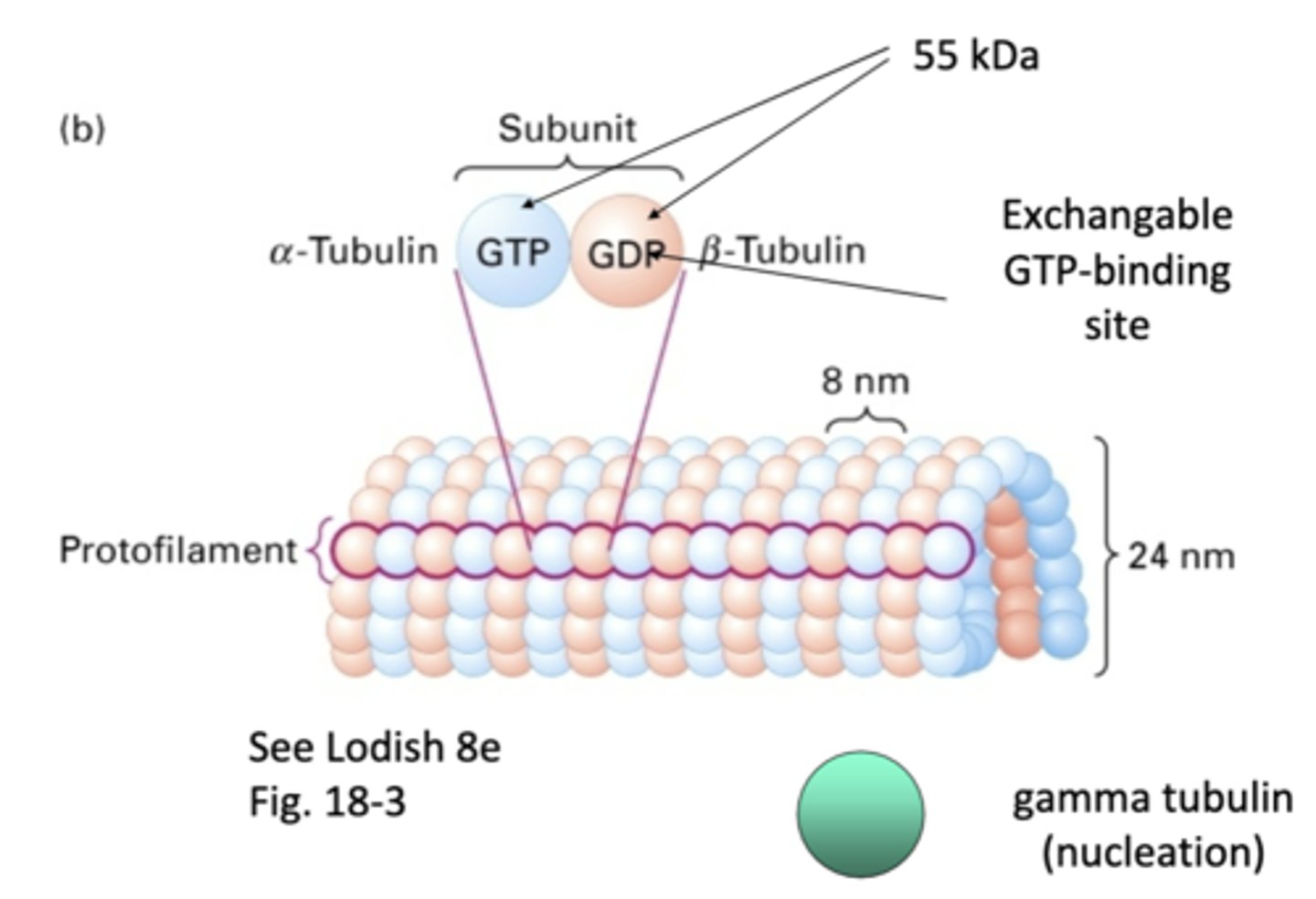
"one strand" of repeating subunits for a microtuble
protofilament
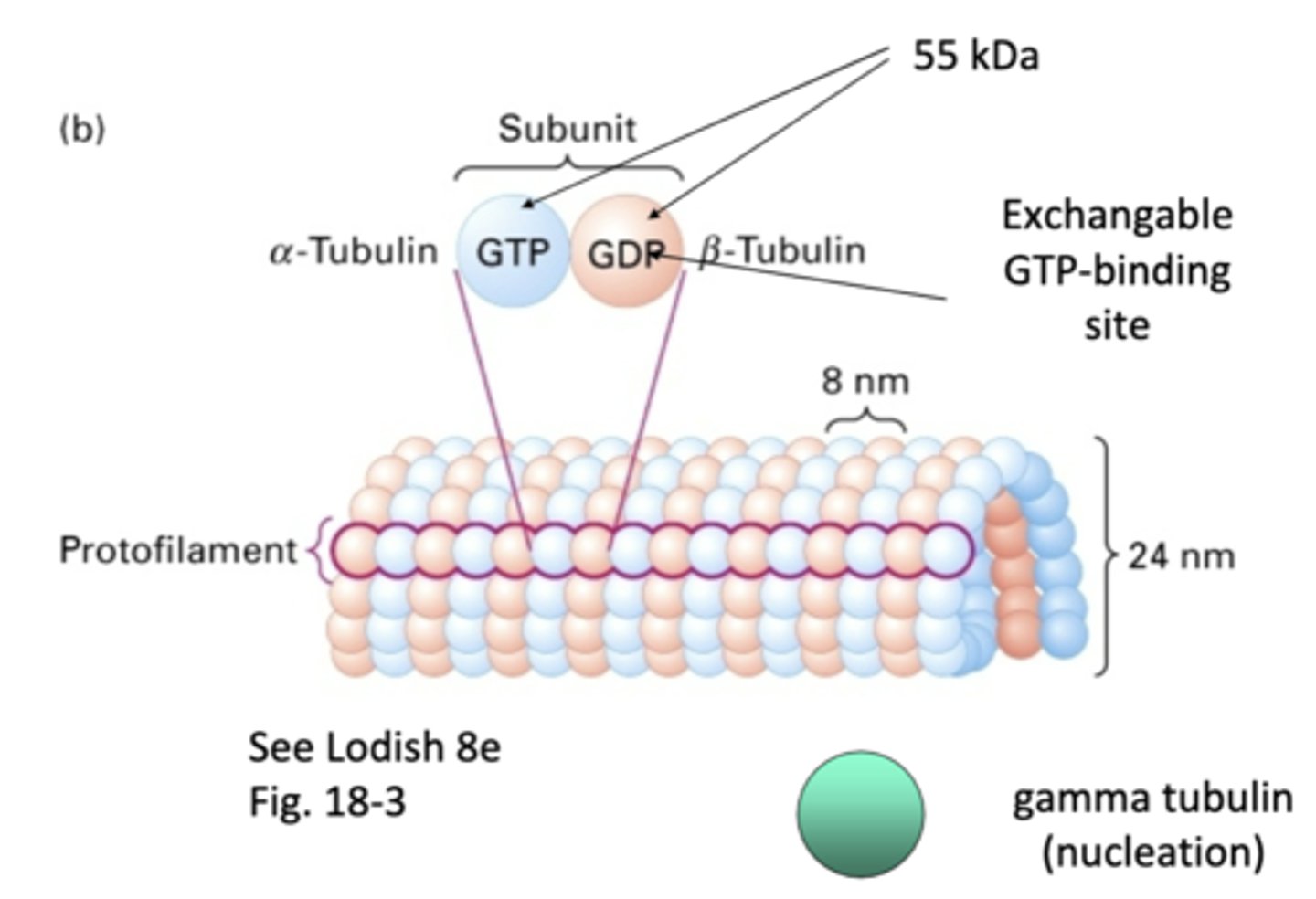
55 kDa
what is the weight of α-tubulin and β-tubulin?
involved in nucleation of microtubules, doesn't become part of final structure
gamma tubulin
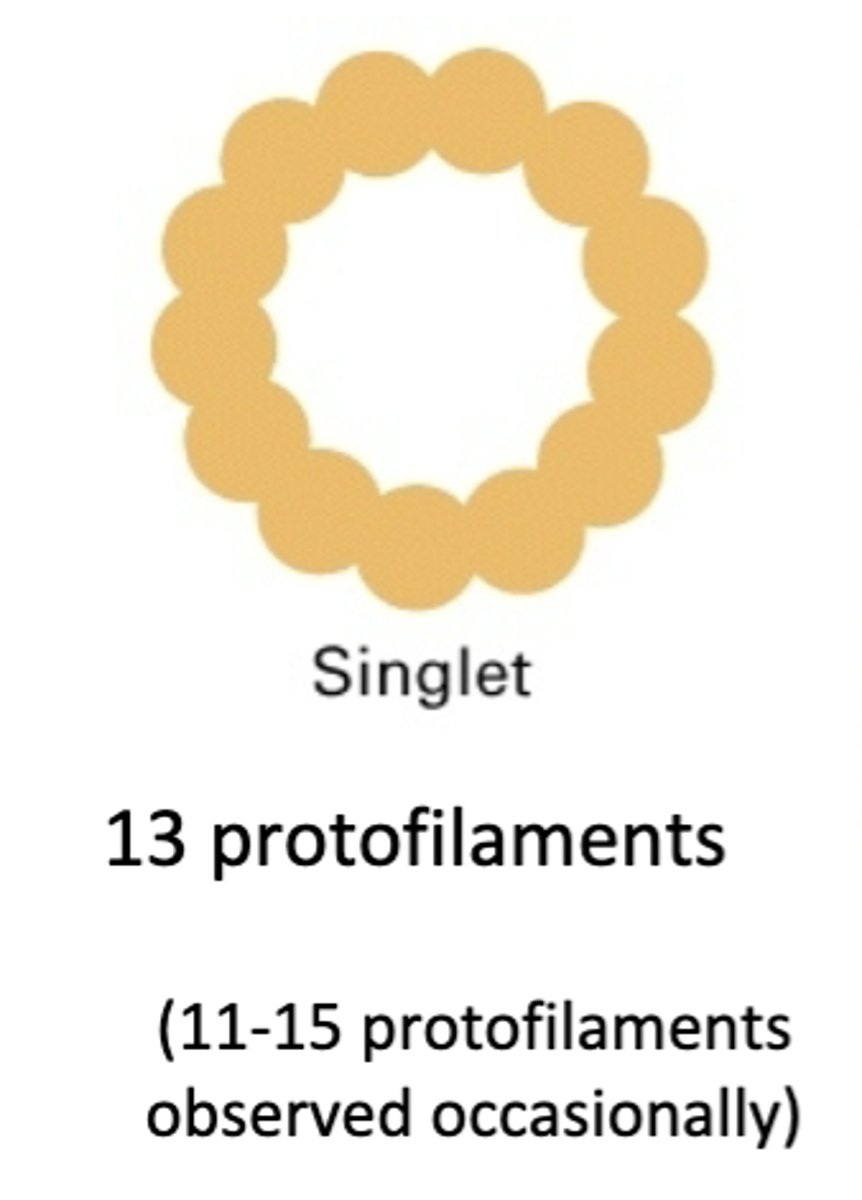
13 protofilaments, majority of microtubules in cytoplasm (11-15 protofilaments observed occasionally)
singlet
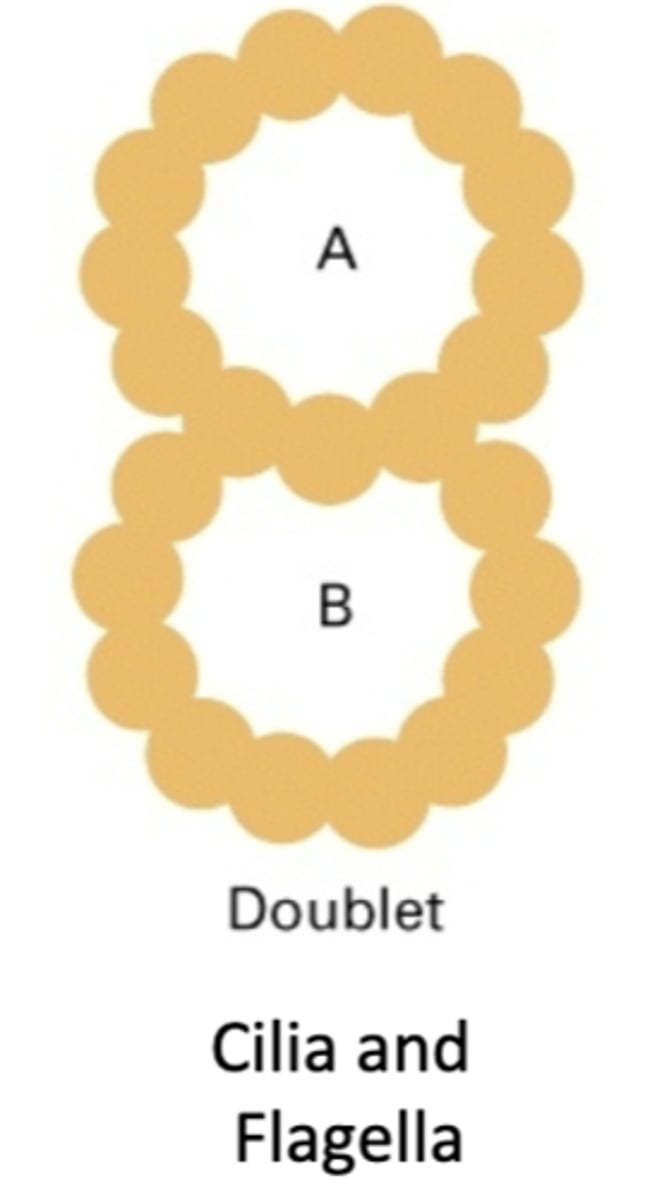
consists of an A tubule and a B tubule, cilia and flagella. 23 protofilaments
doublet
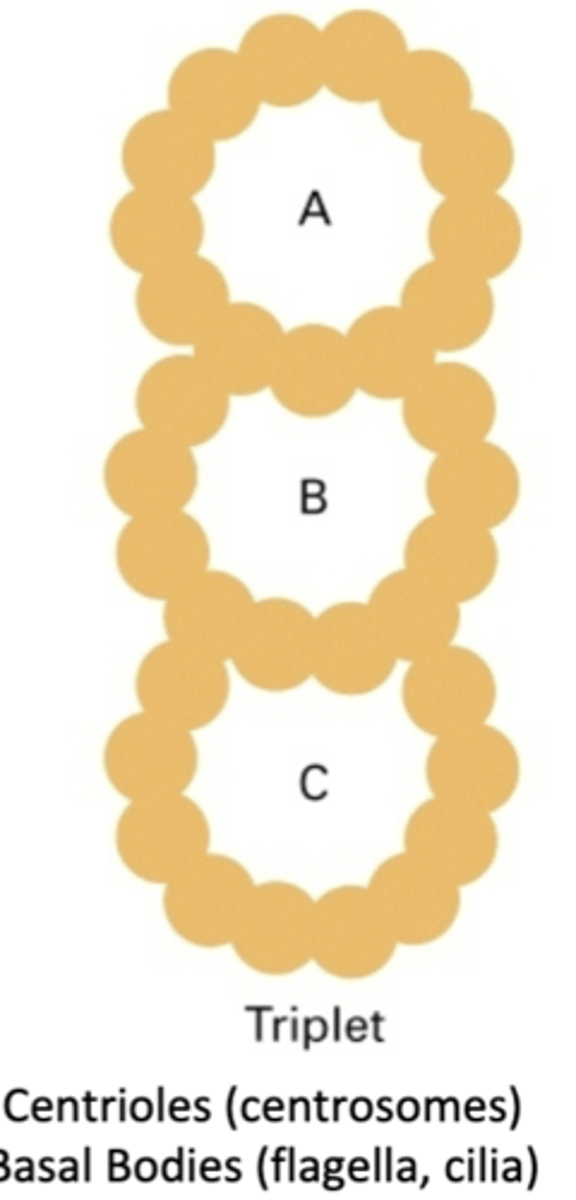
consists of an A tubule, B tubule, and C tubule. centrioles (centrosomes), basal bodies (flagella, cilia), MTOC. 33 protofilaments
triplet
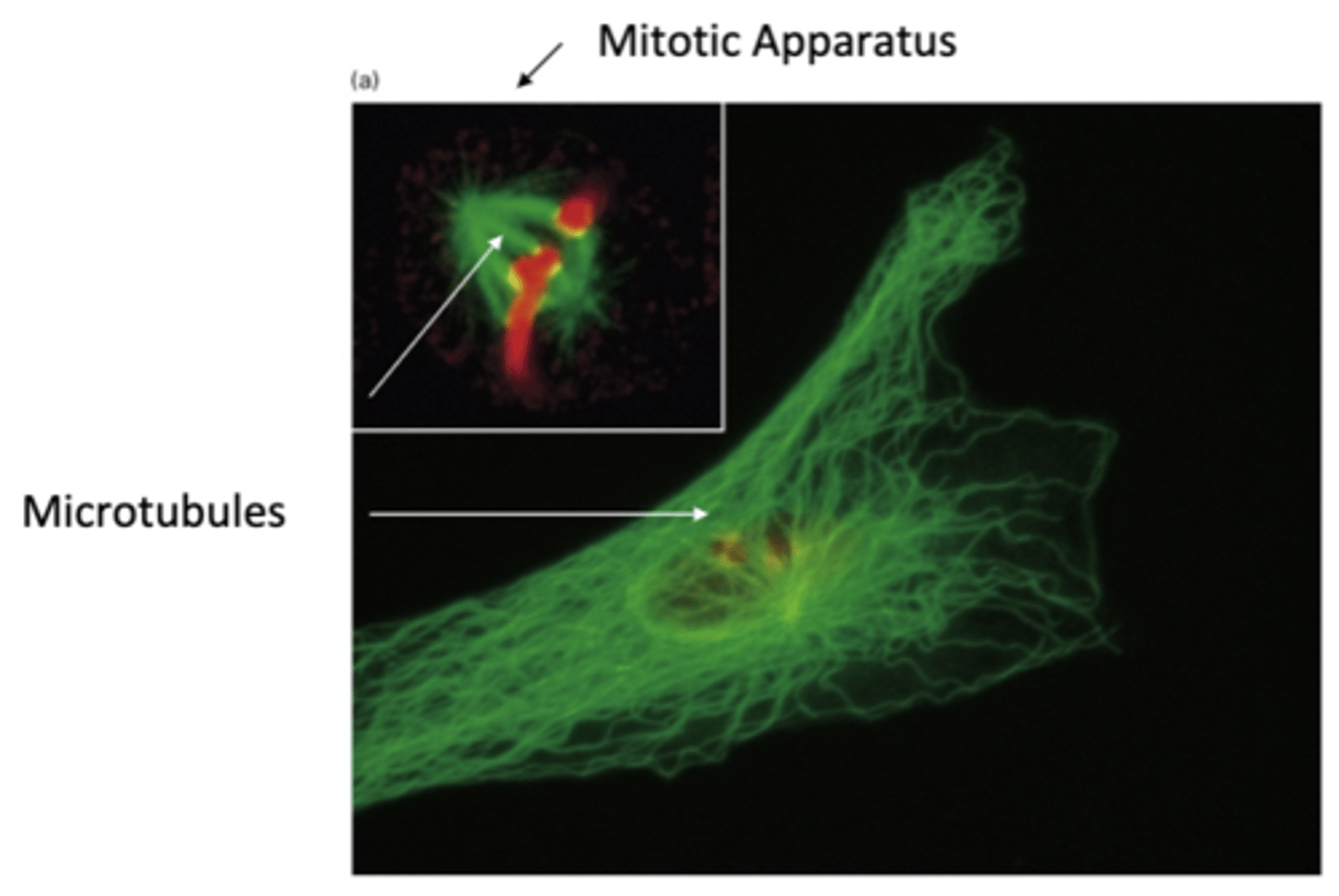
1. unstable, short lived - assembles and disassembles rapidly
2. stable, long lived - remain polymerized for long time
2 populations of microtubules
need to re/depolymerize as cell needs it. example is cell division microtubules in mitotic apparatus
unstable, short lived MT
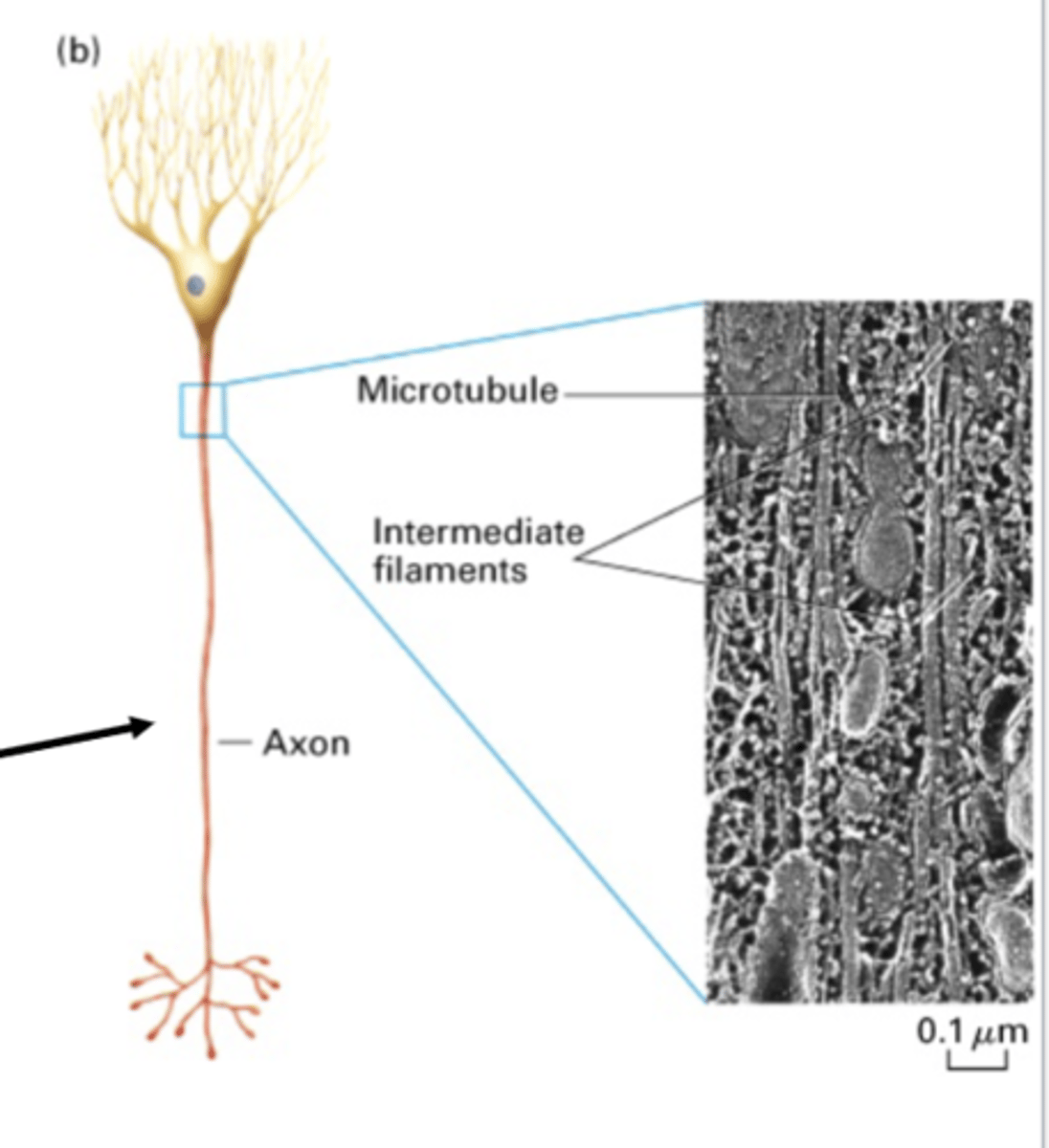
maintain structural integrity of cell. examples are sperm flagella, red blood cells (marginal band), platelets, nerve cells (axons and dendrites)
stable, long lived MT
intermediate filaments
in a nerve axon, microtubules work together with ________________ ___________________
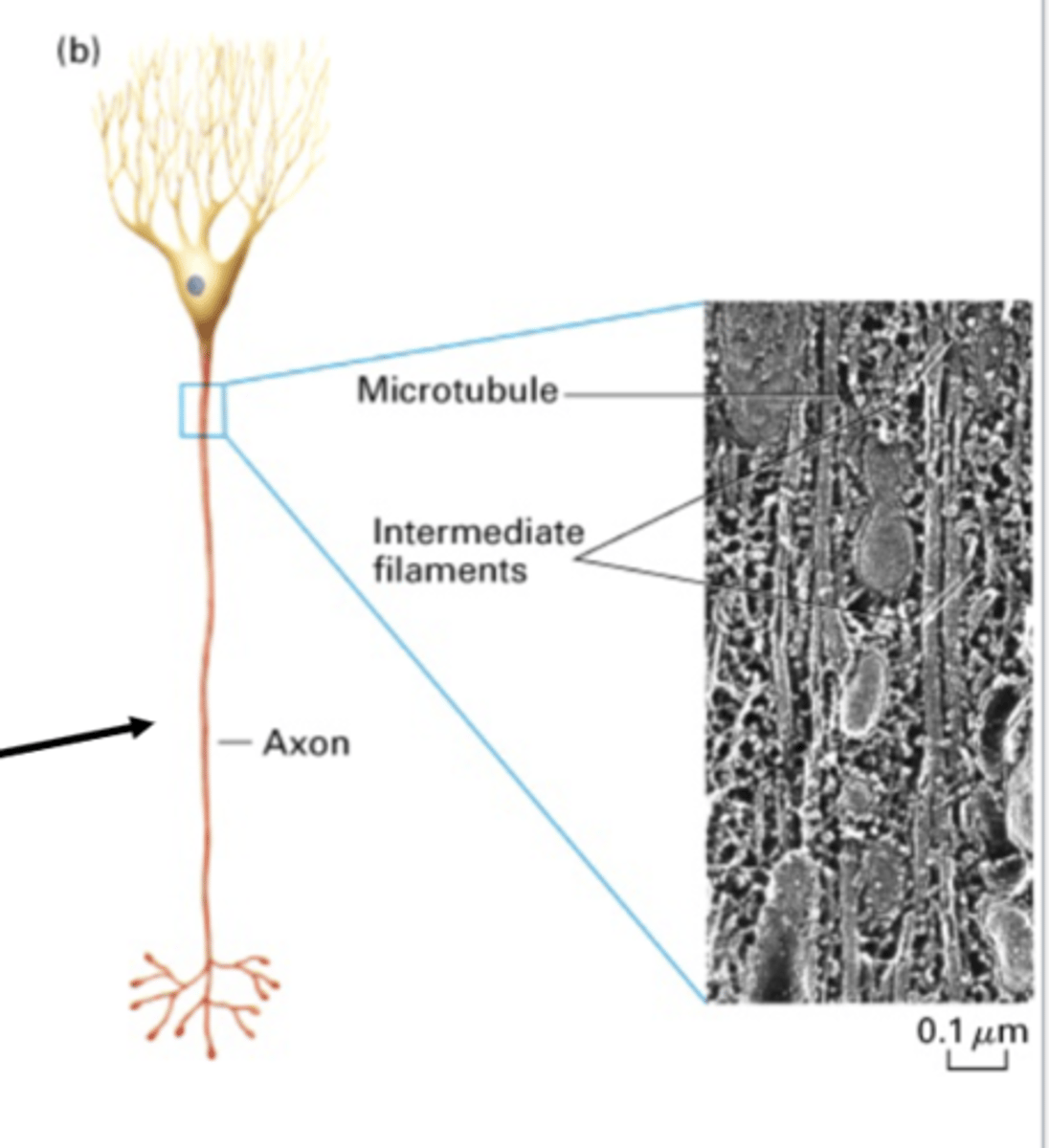
run almost along-side MT and wrap around nuclei for integrity
in a nerve axon, intermediate filaments do what?
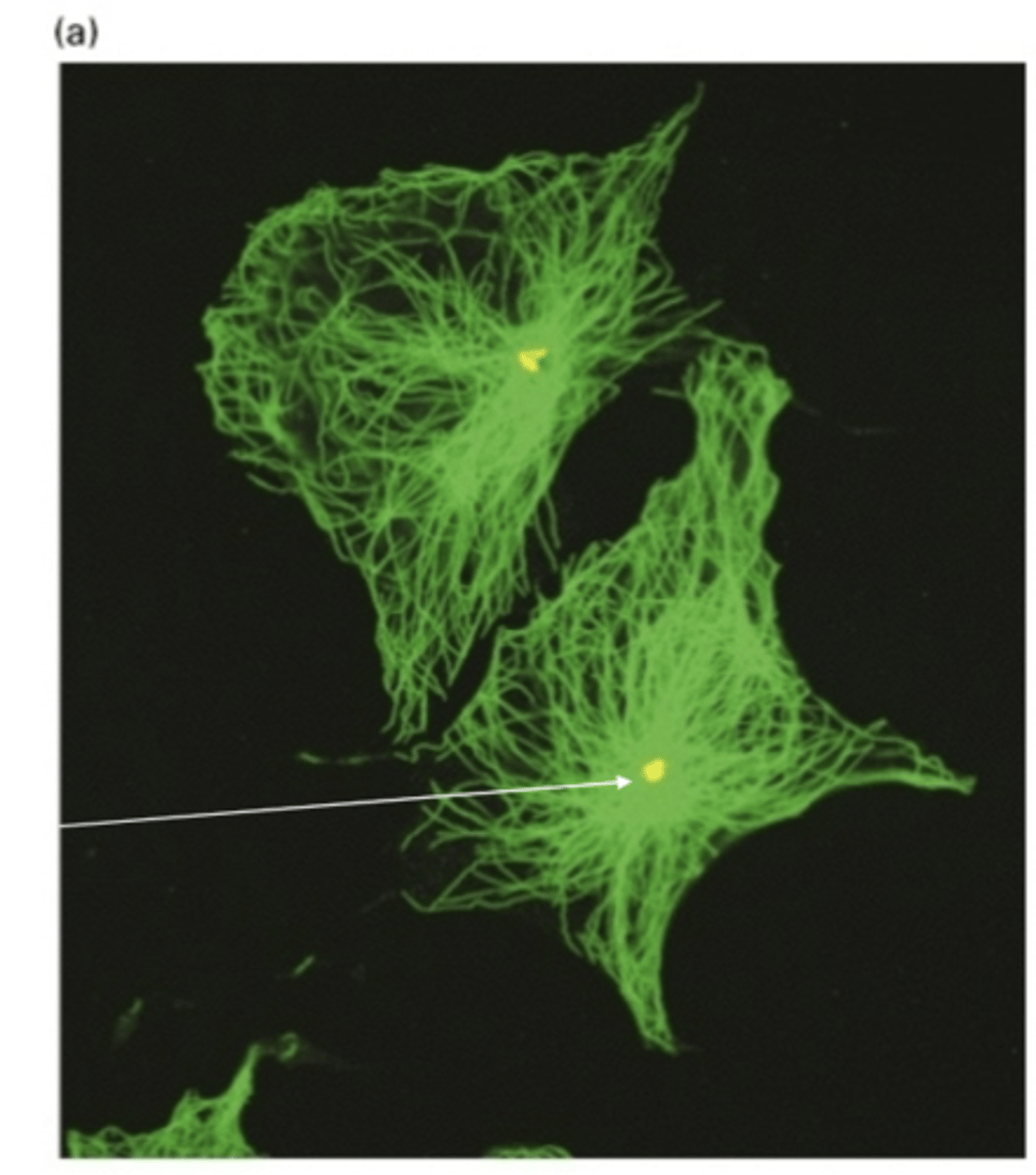
any structure used by cells to nucleate and organize MT (lots of different kinds)
microtubule organizing center (MTOC)
centrosome
primary MTOC in animal cells
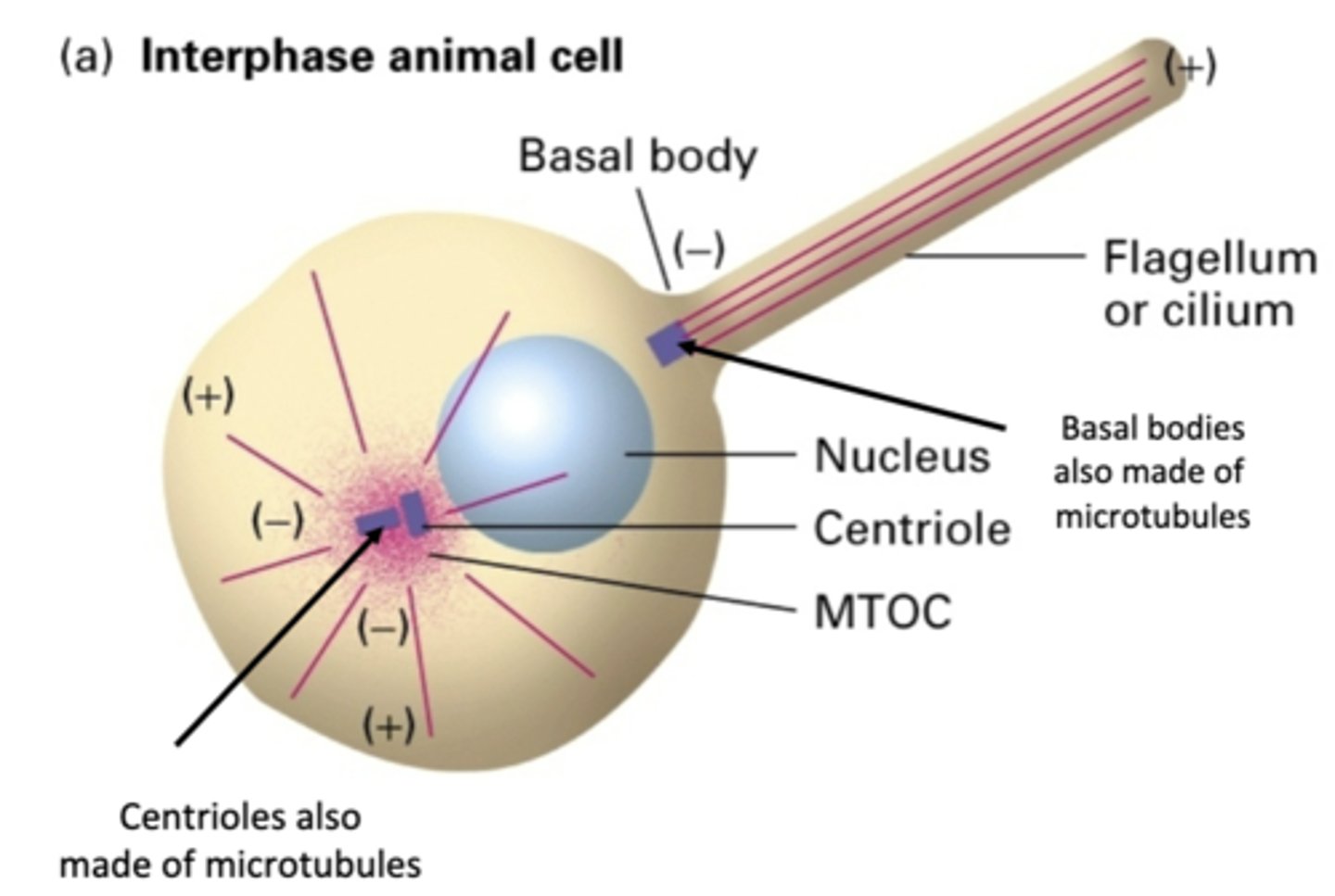
basal body
what organizes MT in flagella?
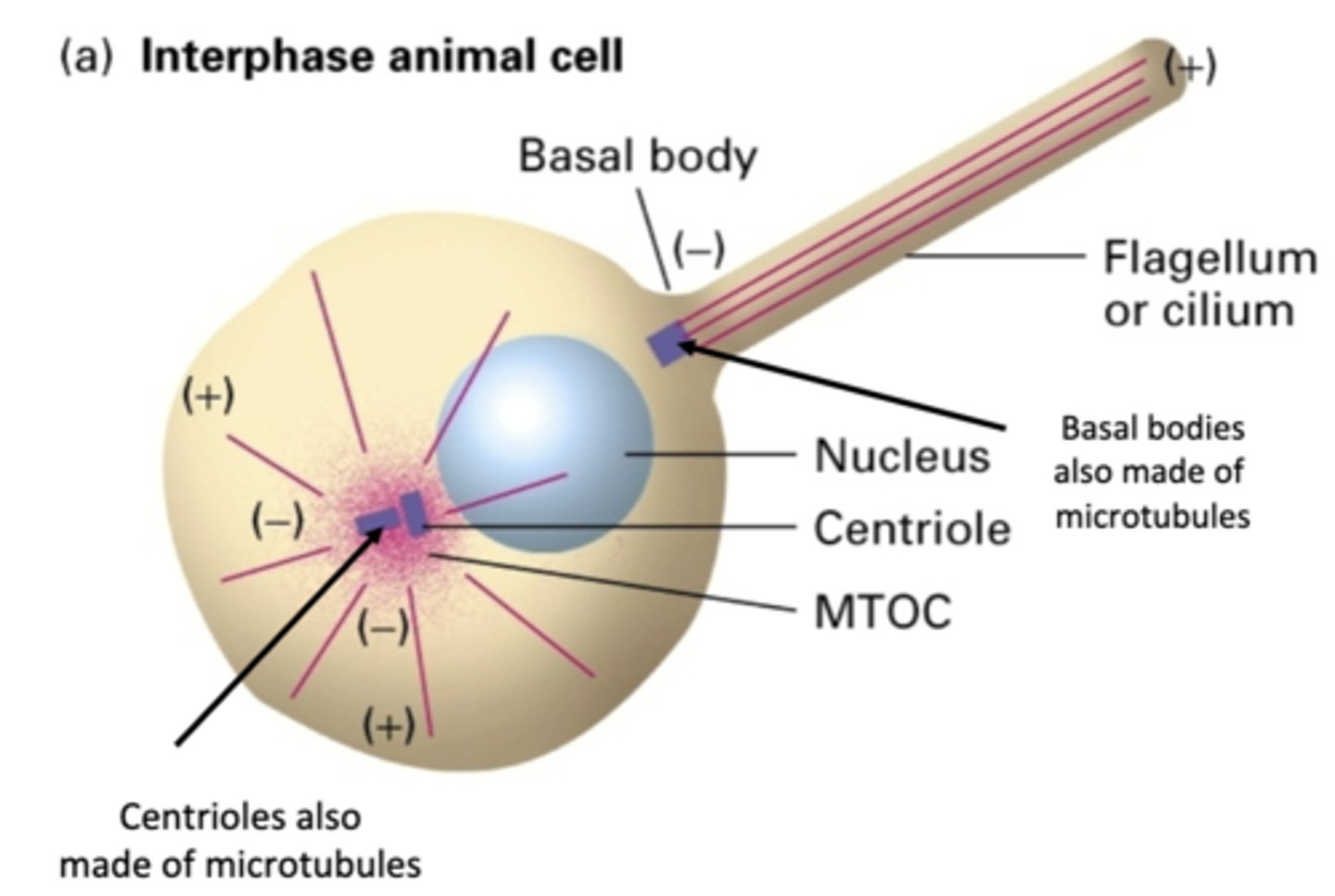
microtubules
basal bodies and centrioles are made of ____________________
-, +
MT have a ___ end and a ___ end
polar
because of the different ends of MT, MT are said to be _________________
-
in MT emanating from centrioles, the ___ end of MT are bound to organizing center
+
in MT emanating from centrioles, the ___ end of MT are furthest from the center
inside centrosome, very short triplet MT
centriole
centrioled
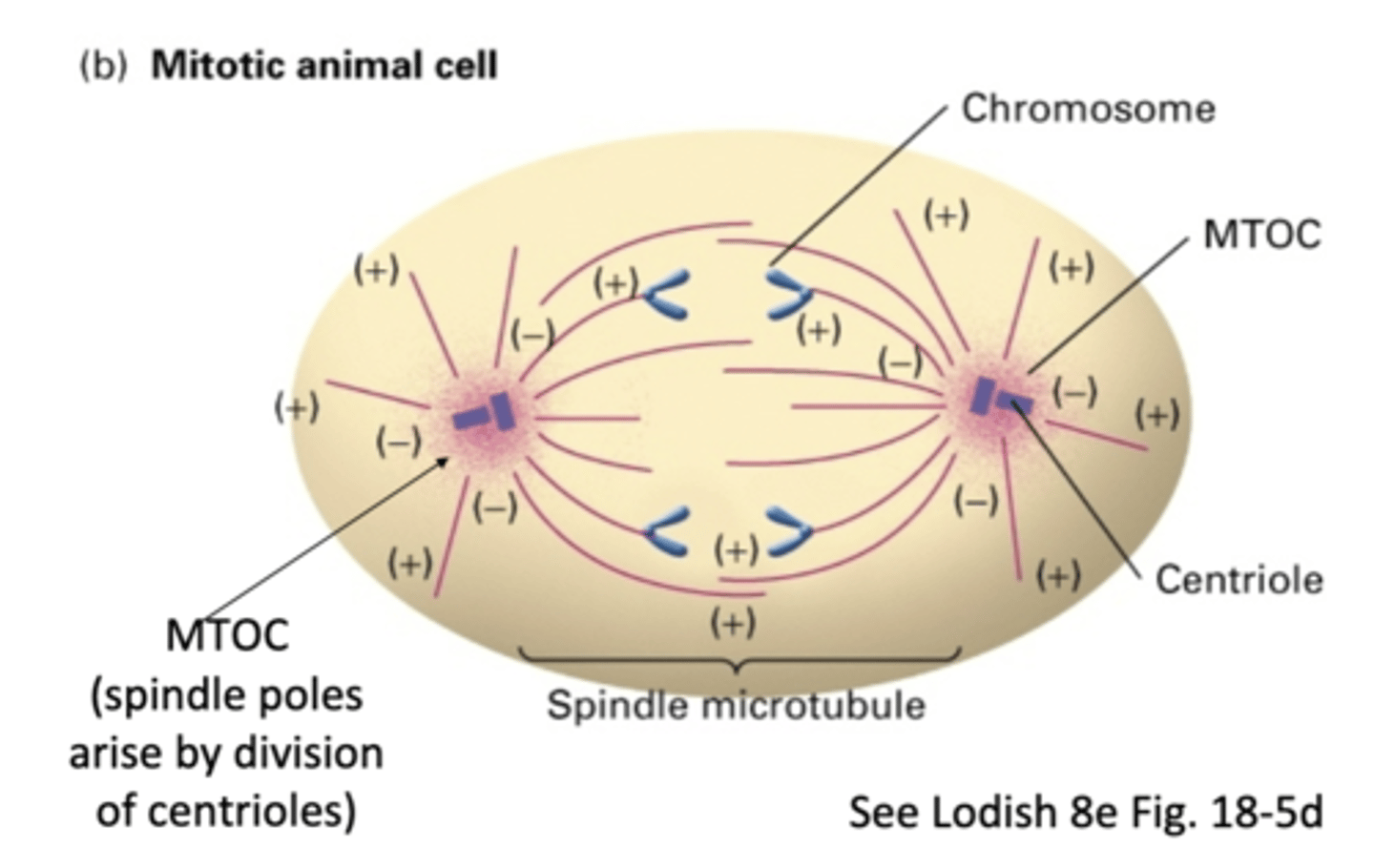
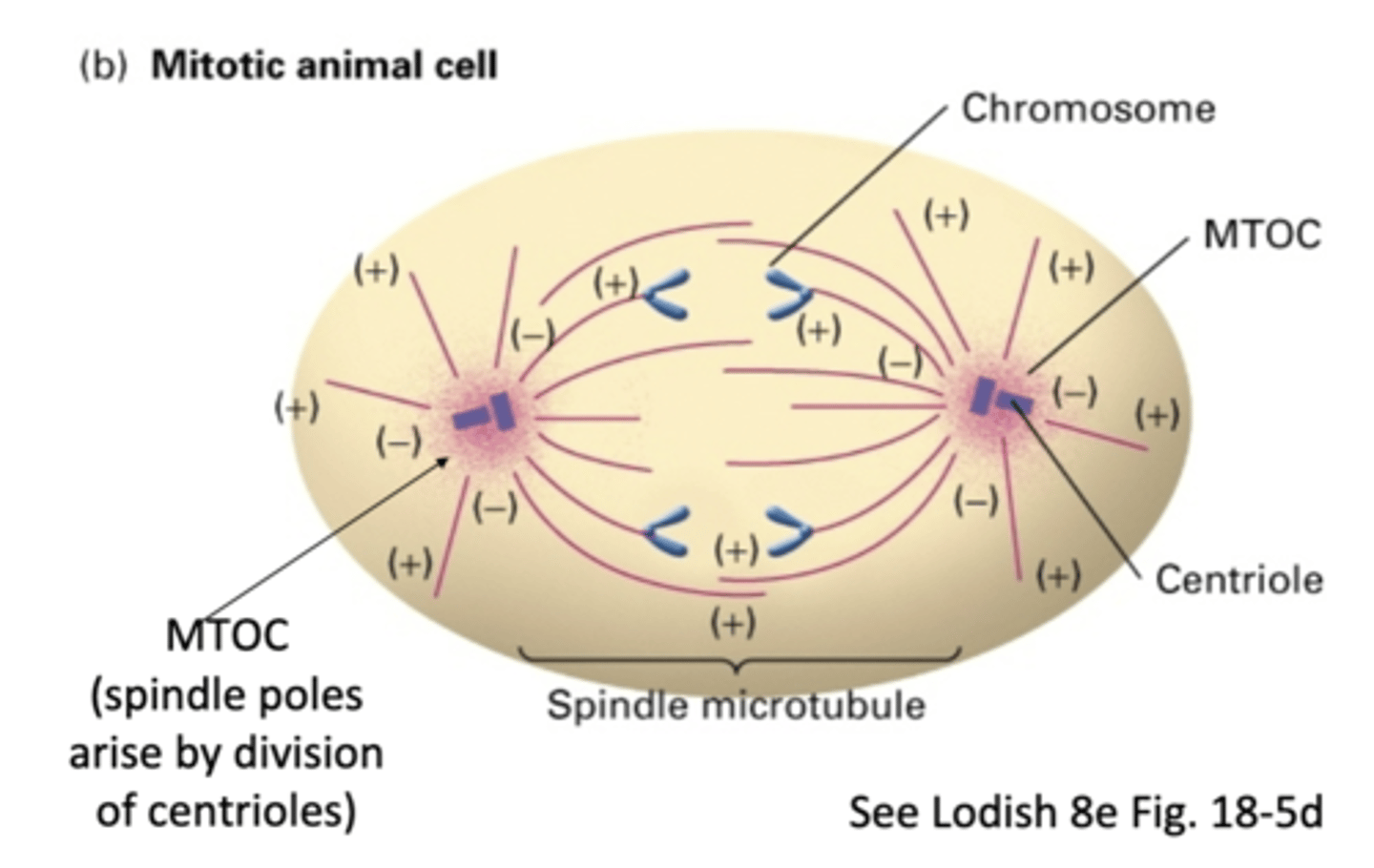
the spindle poles arise by division of _______________
different
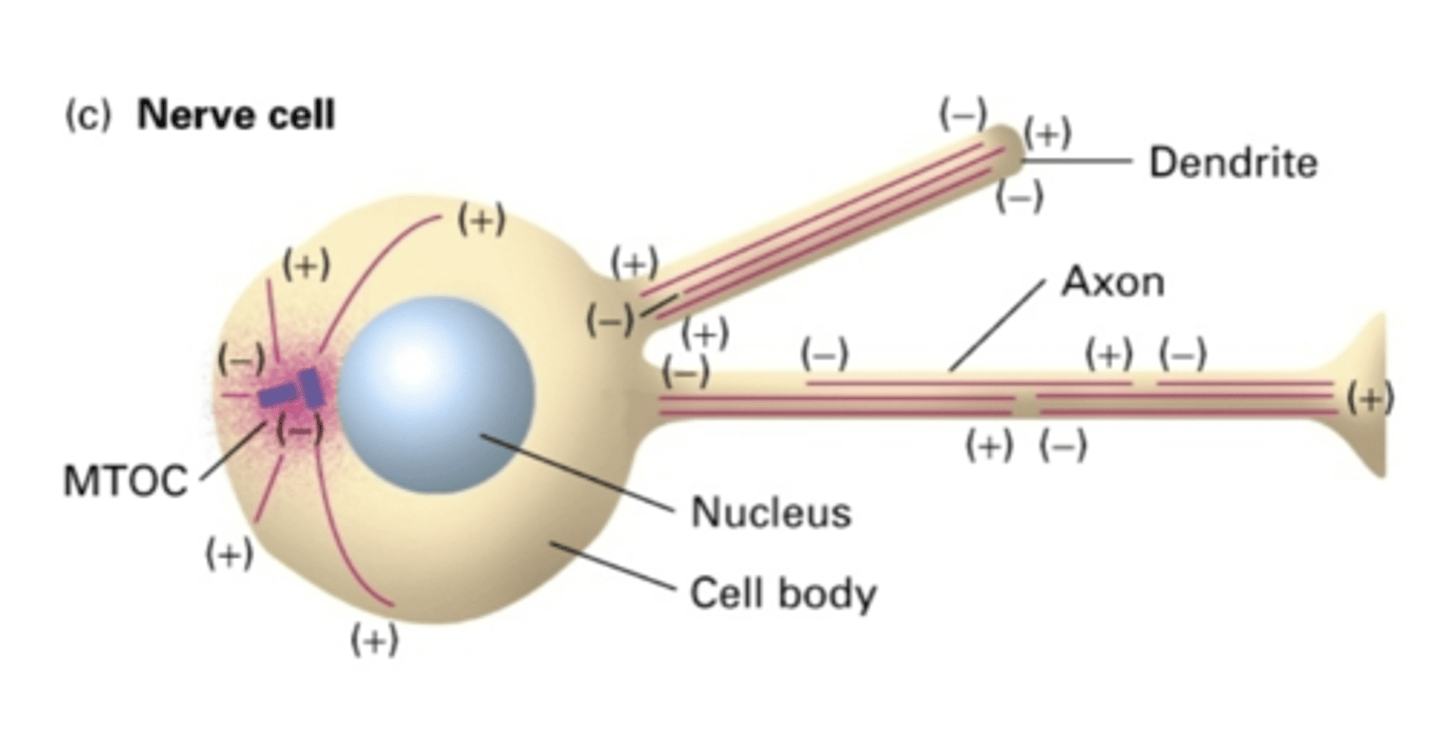
in the dendrites of nerve cells, the microtubules are in (same/different) directions
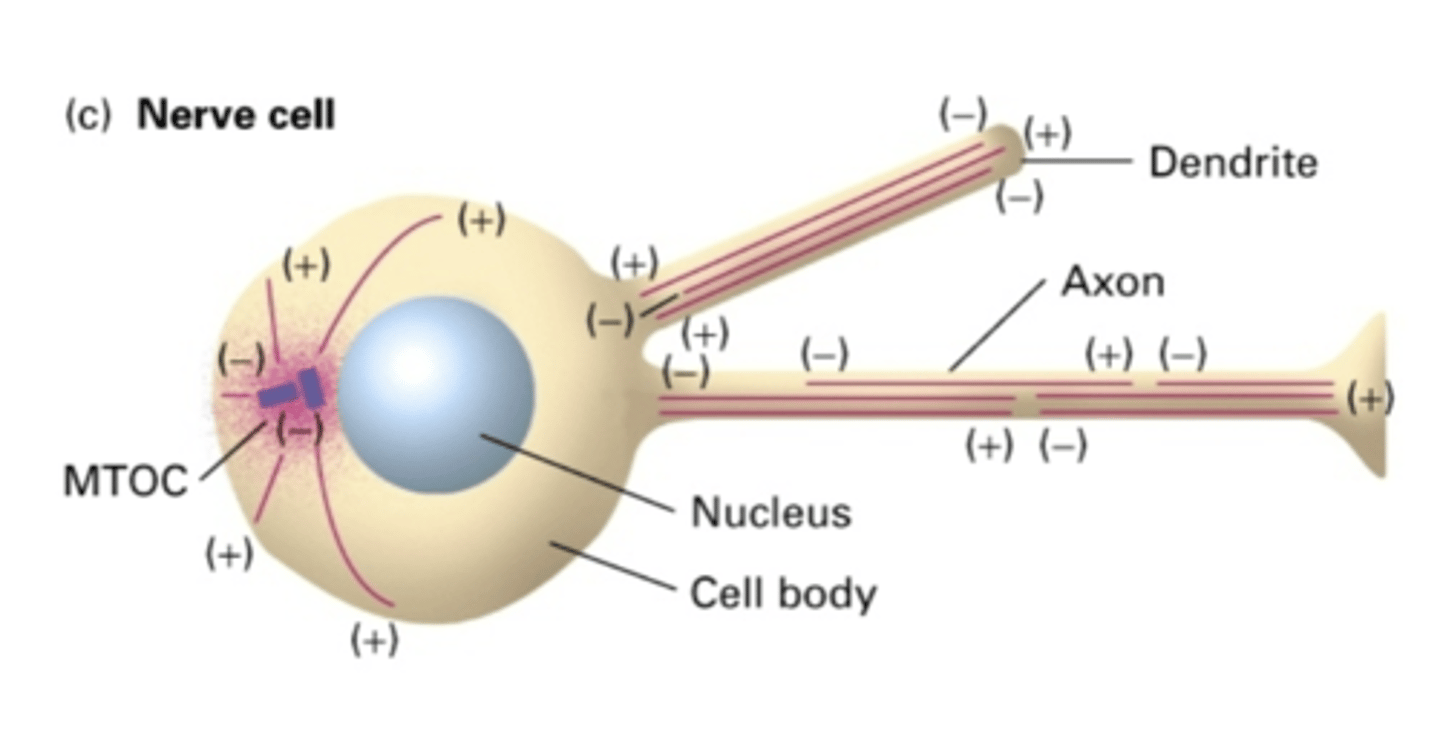
same
in the axon of nerve cells, the microtubules are in (same/different) directions
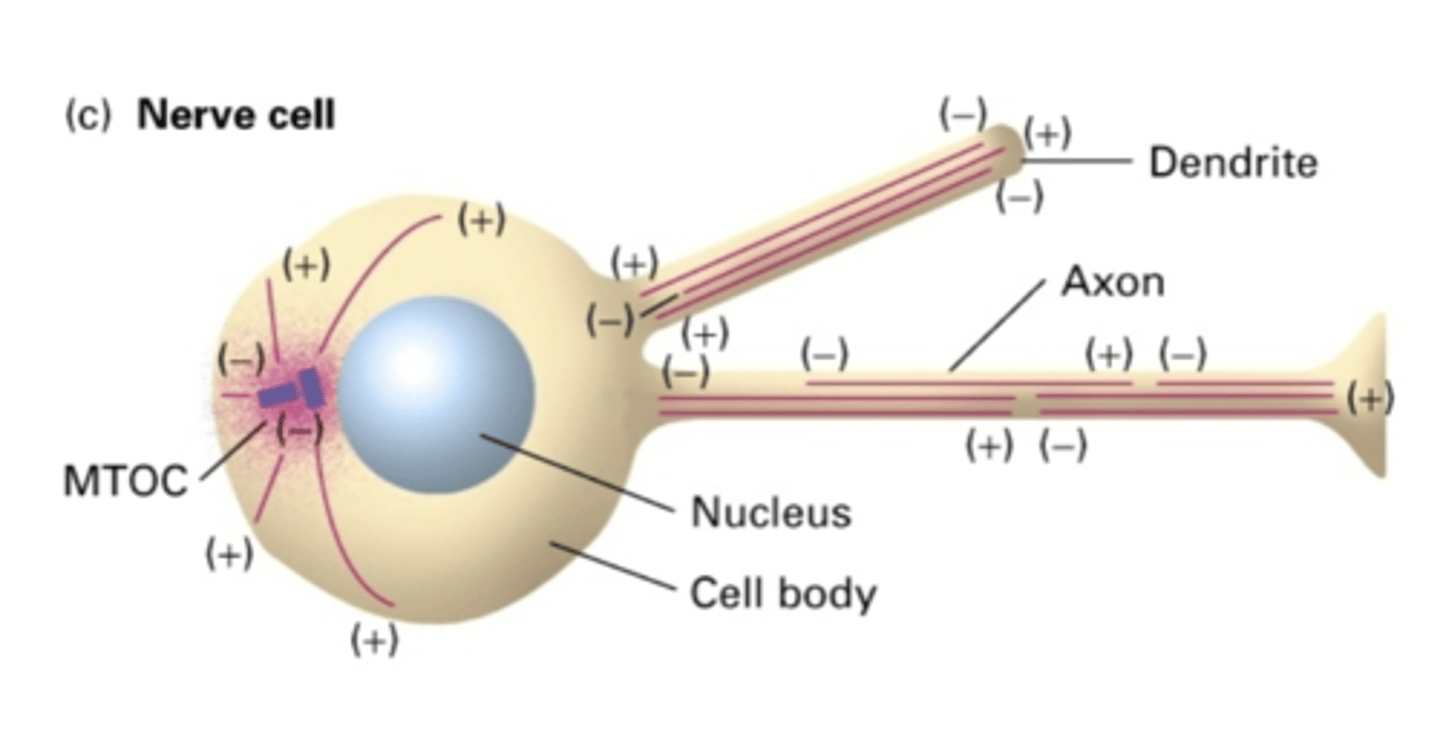
NO
do nerve cells use a MTOC?
YES - but not in same orientation
in the dendrites of nerve cells, do MT extend the whole length of the dendrite?
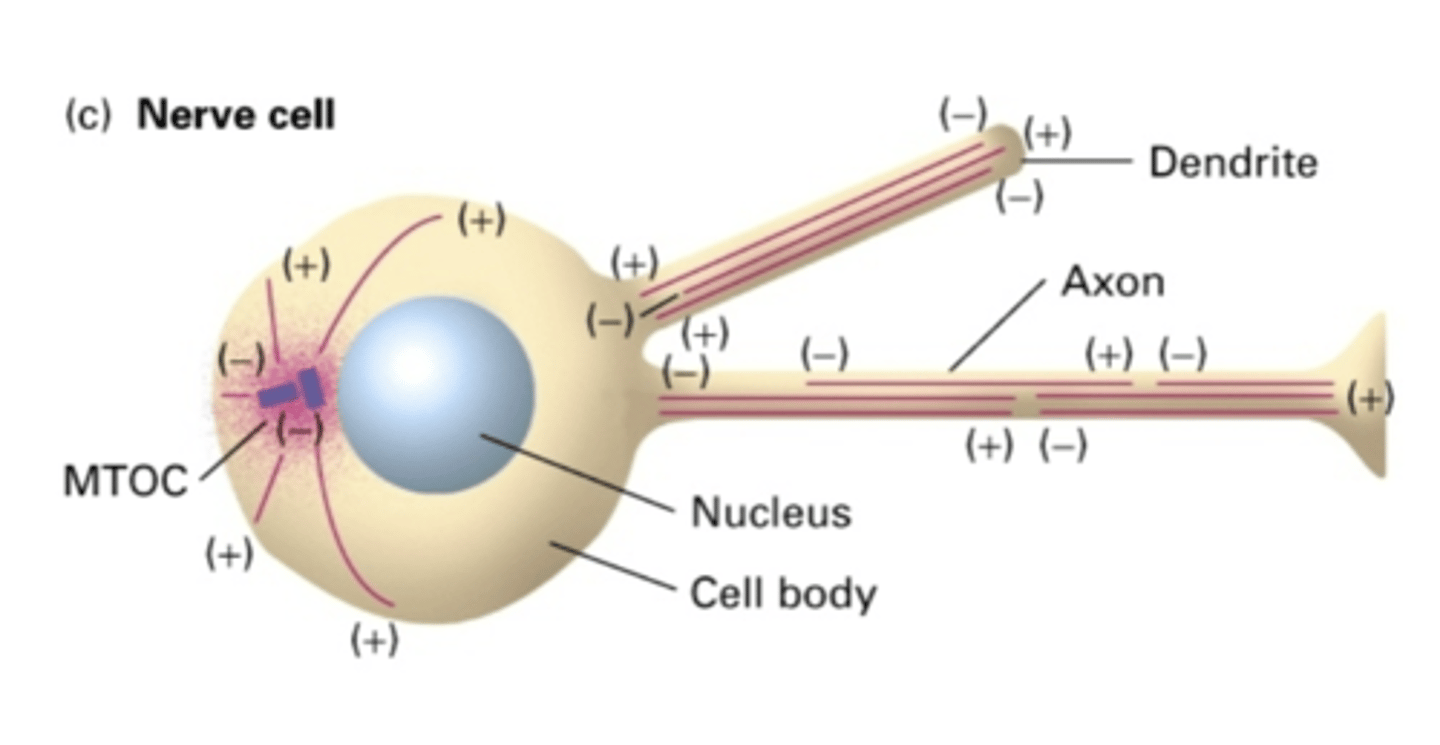
NO - but in same orientation (-) -> (+)
in the axon of nerve cells, do MT extend the whole length of the axon?
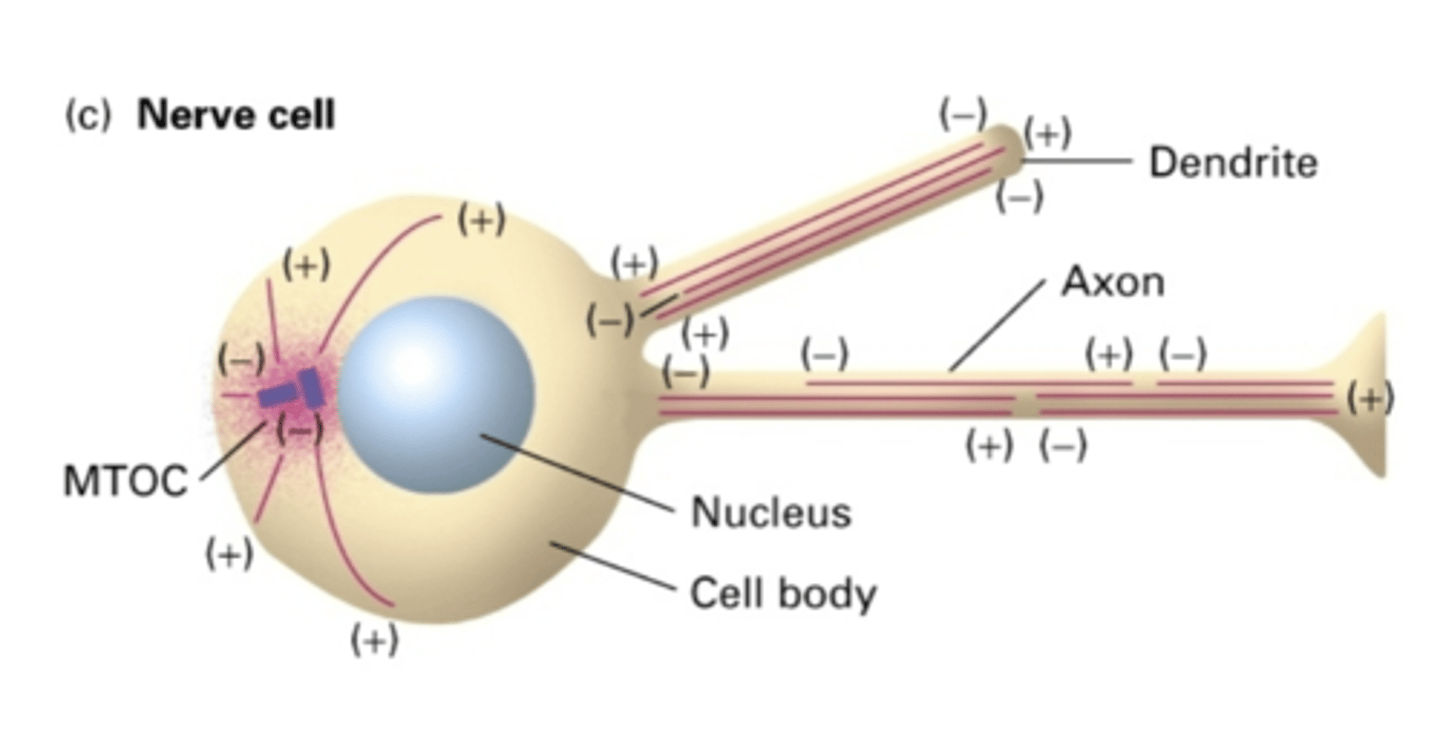
the cell body
in the axon of nerve cells, the (-) end is facing where?
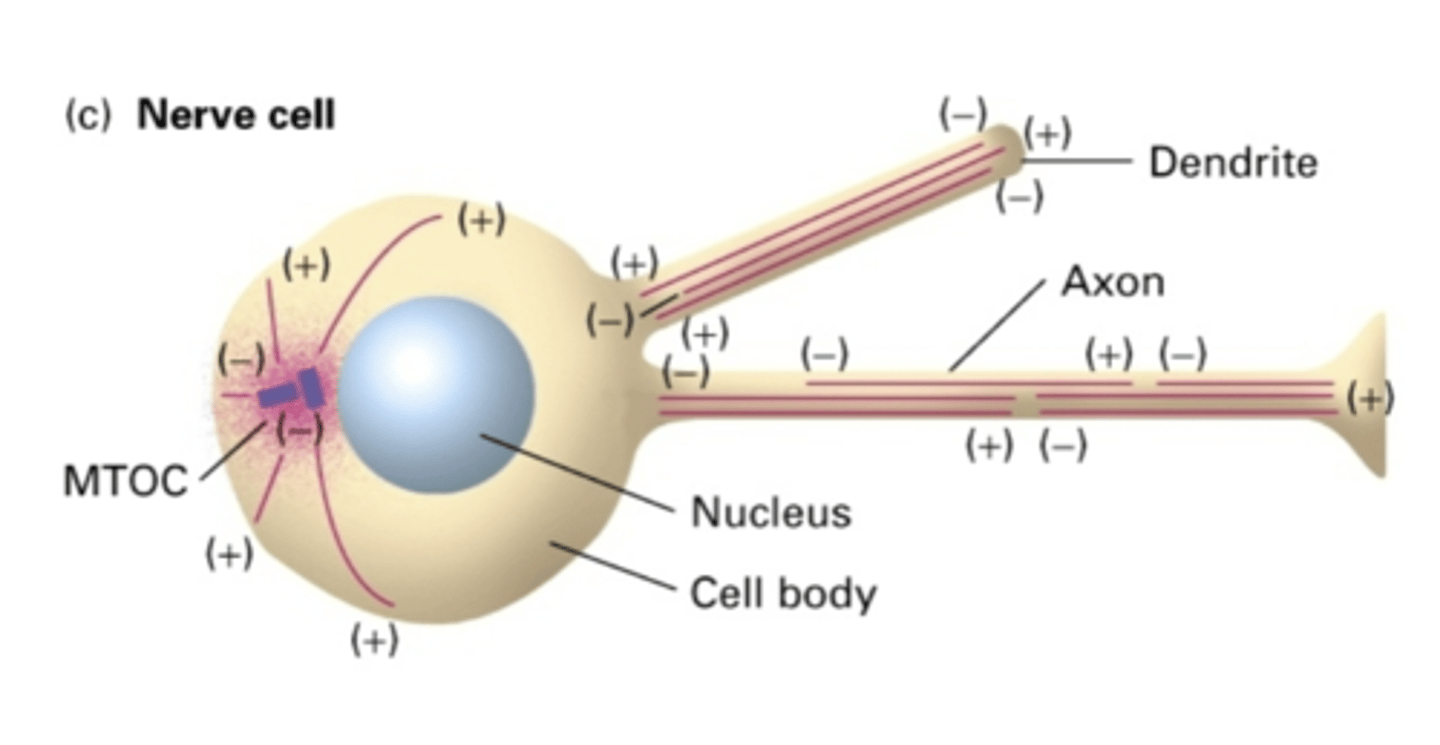
the length of the axon
in the axon of nerve cells, the (+) end is facing where?
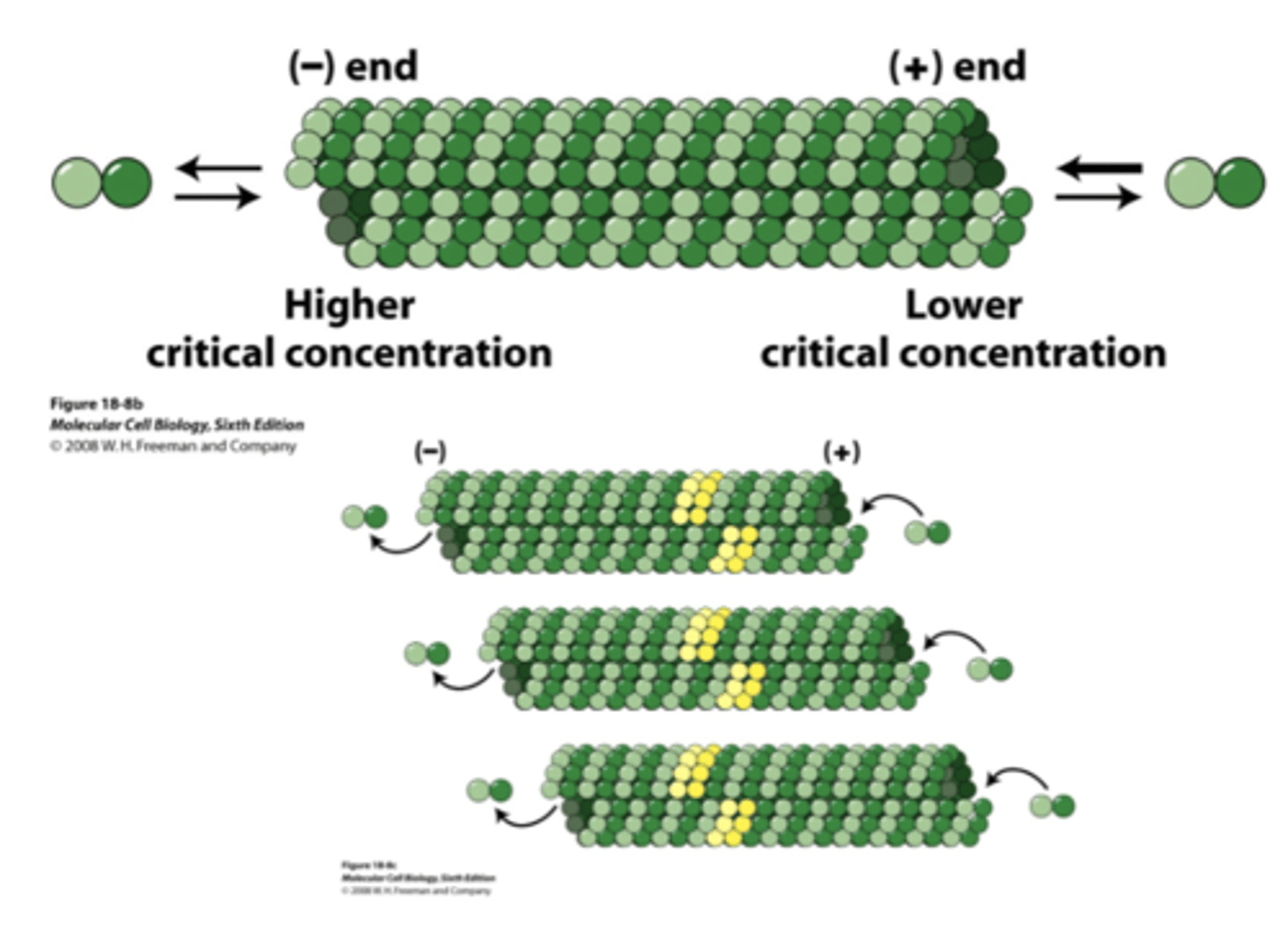
lower, higher
in MT, the (+) end has a (higher/lower) critical concentration while the (-) end has a (higher/lower) critical concentration
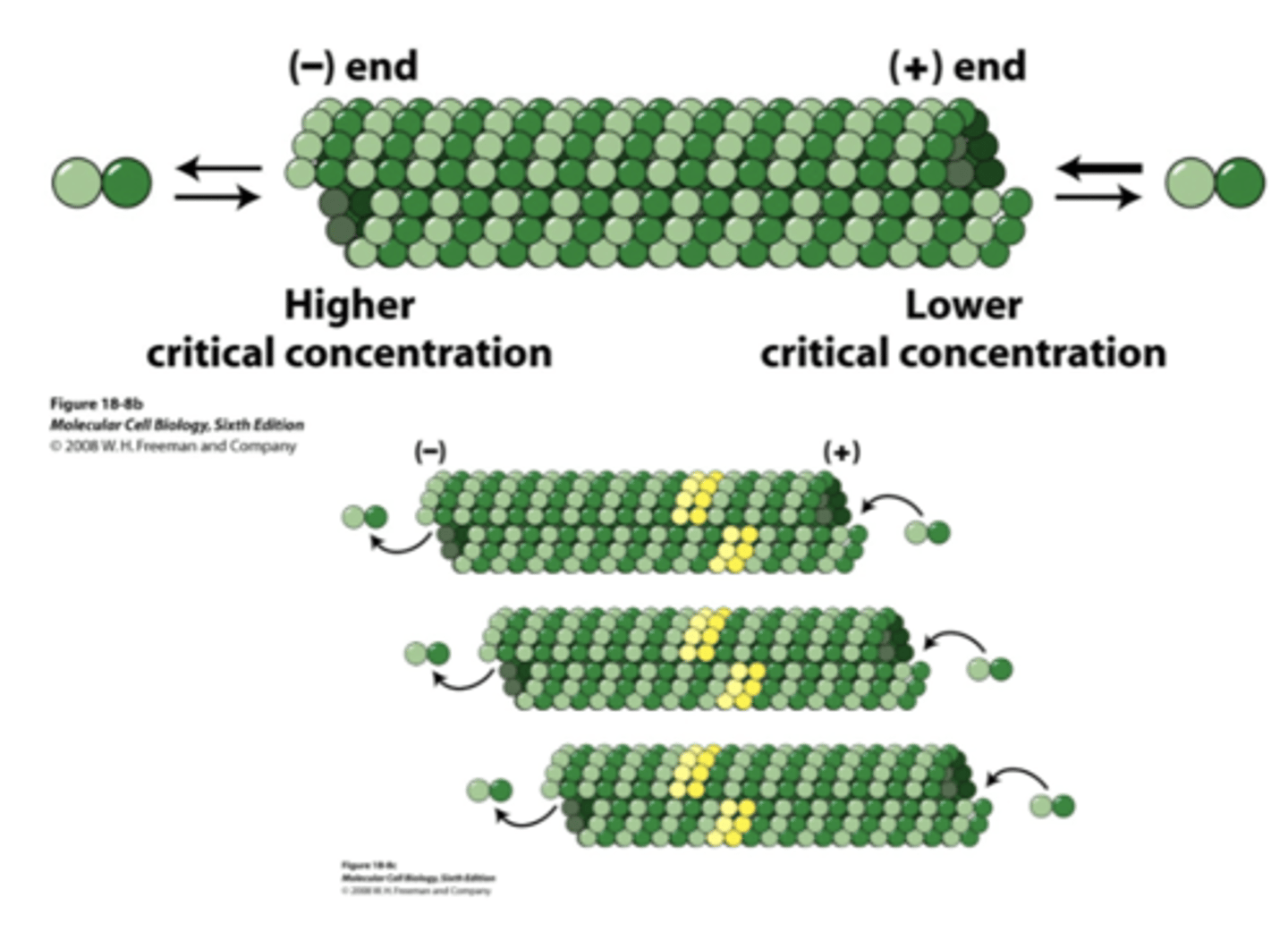
treadmilling, addition, loss
_____________________________ occurs along the length of MT due to (addition/loss) at the (+) end and (addition/loss) at the (-) end
depolymerization of MT
if a cell is in interphase, what will happen with colcemid is added or cooled to 0 degrees C?
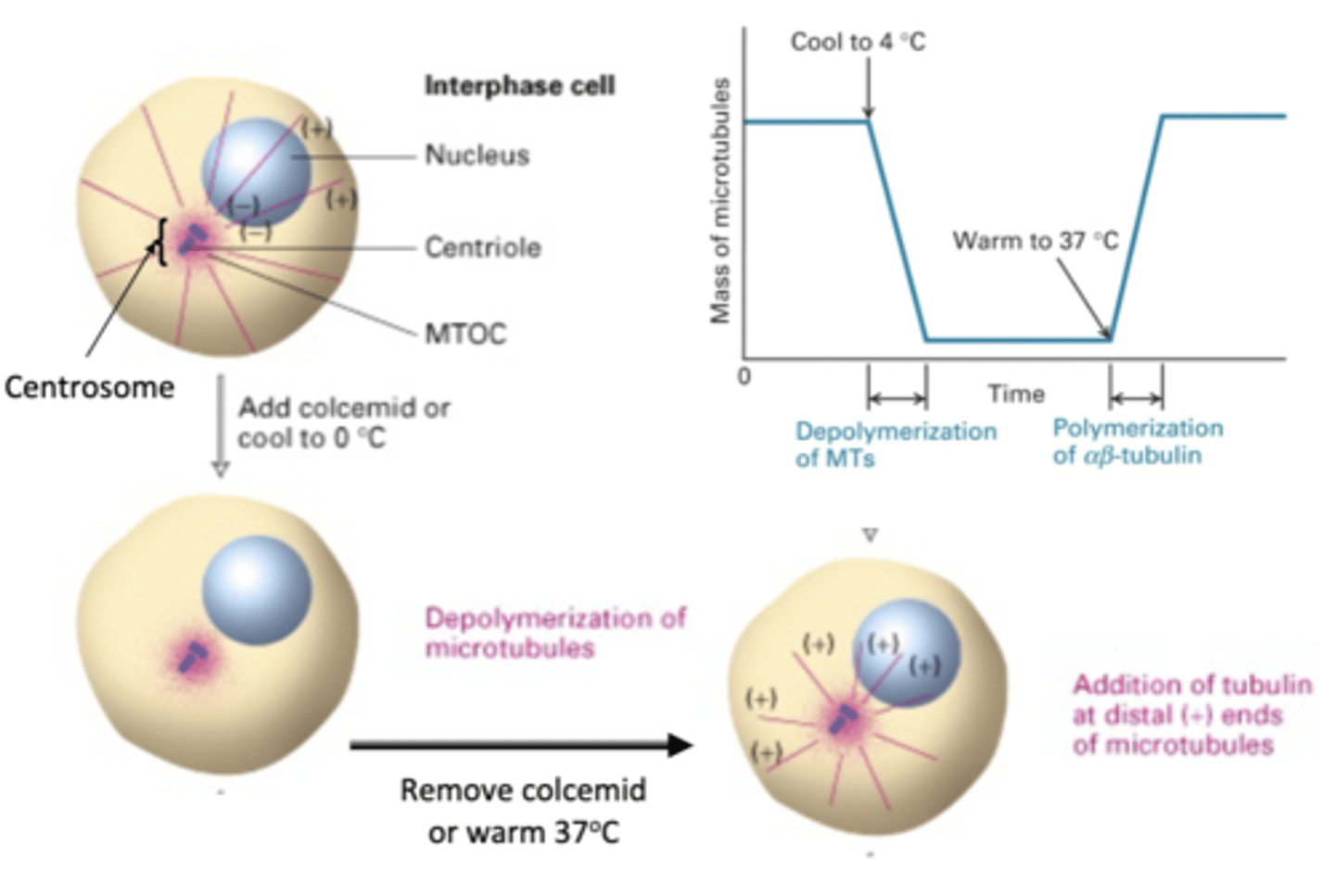
re-polymerization of MT
what will happen is colcemid is removed or the cell is warmed to 37 degrees C?
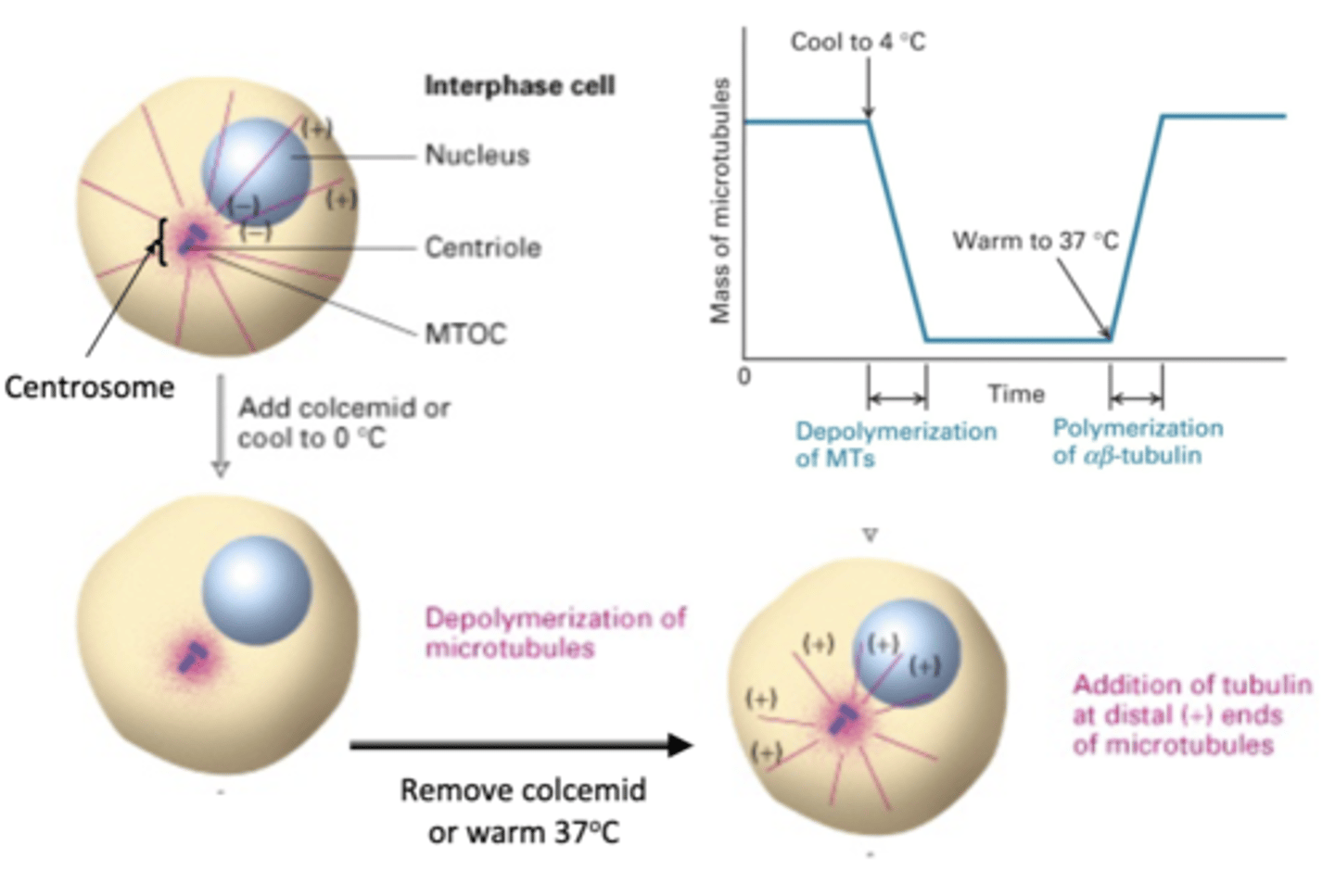
reversible
the depolymerization/repolymerization experiment shows that this process is (permanent/reversible)
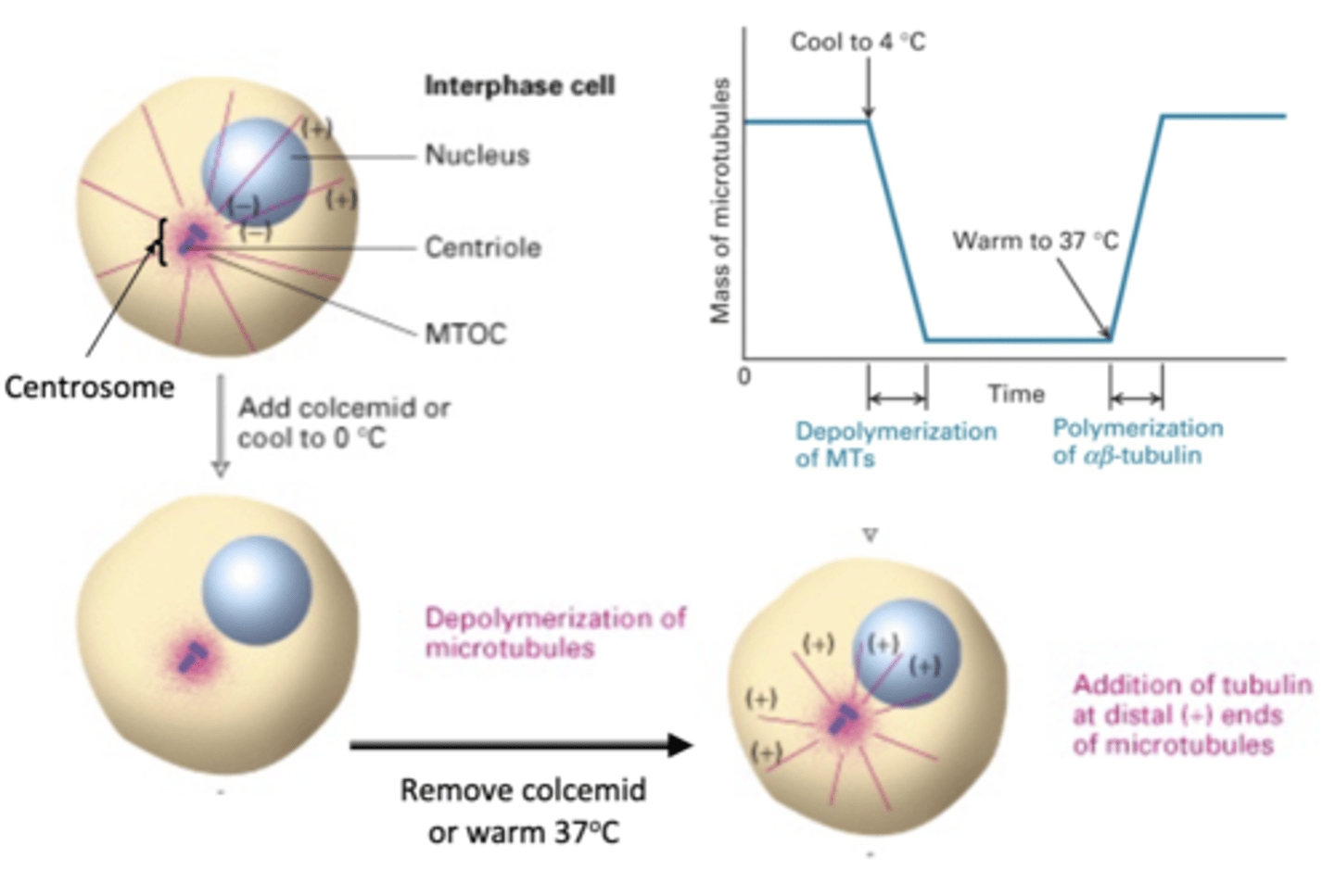
nucleate
MTOC can ______________ MT
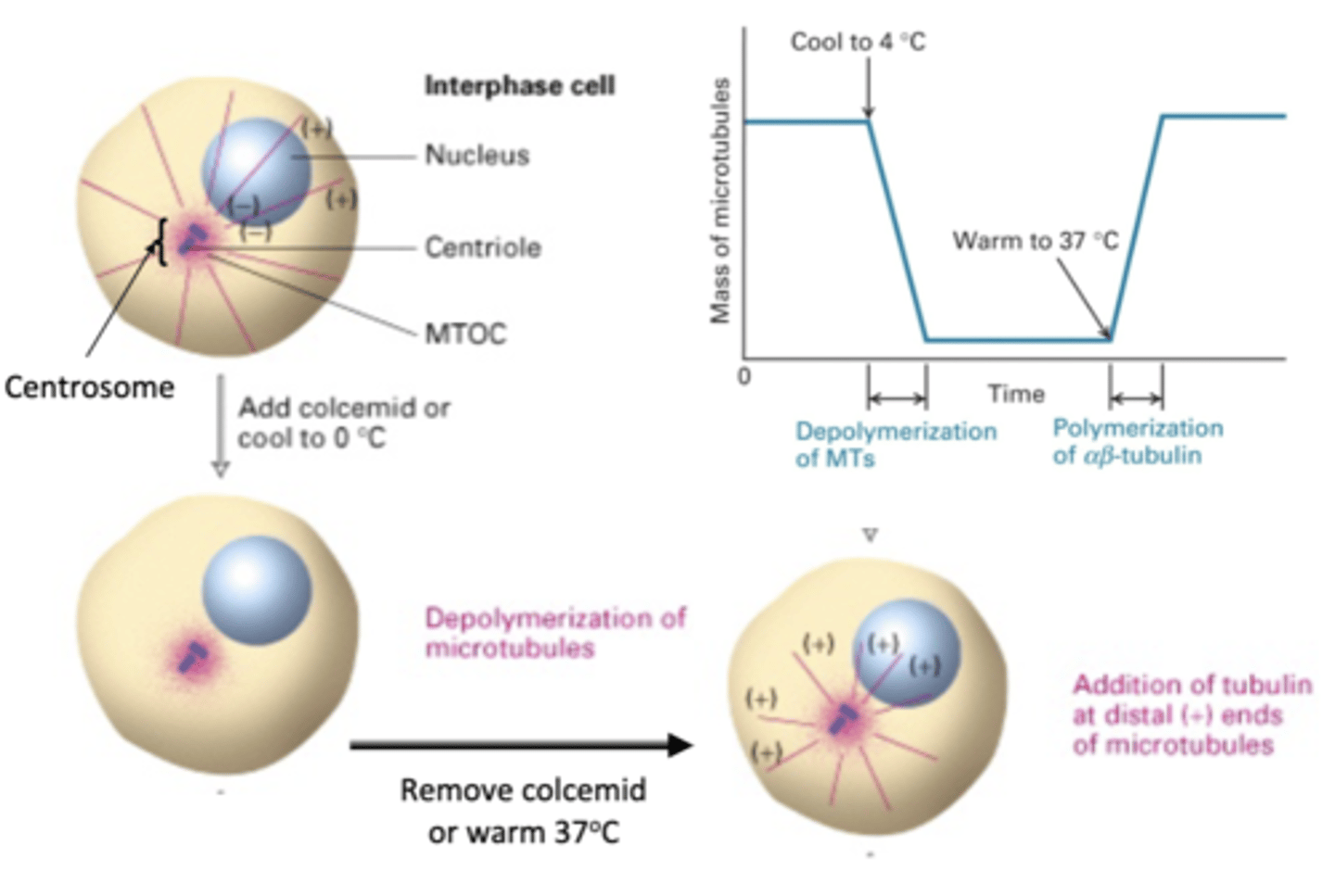
BOTH: yes (+ and - ends)
microfilaments vs. microtubules: polar
microfilaments: Yes-ATP
microtubules: Yes-GTP
microfilaments vs. microtubules: binding nucleotide
actin-ATP (in vivo)
actin-ADP (in vitro)*
Actin-non hydrolyzable ATP analogs (in vitro)*
(**Although triphosphate nucleotides (ATP or GTP) are required for subunits to polymerize in vivo, it is possibleto force subunits bound to diphosphate nucleotides (ADP or GDP) or to non-hydrolysable analogs of ATP/GTP to polymerize in vitro (in the test tube).)
subunits that can polymerize microfilaments
tublin-GTP (in vivo)
tubulin-GDP (in vitro)*
Tubulin-non hydrolyzable GTP analogs (in vitro)*
(**Although triphosphate nucleotides (ATP or GTP) are required for subunits to polymerize in vivo, it is possibleto force subunits bound to diphosphate nucleotides (ADP or GDP) or to non-hydrolysable analogs of ATP/GTP to polymerize in vitro (in the test tube).)
subunits that can polymerize microtubules
microfilaments: ATP hydrolysis required
microtubules: GTP hydrolysis required
microfilaments vs. microtubules: depolymerization
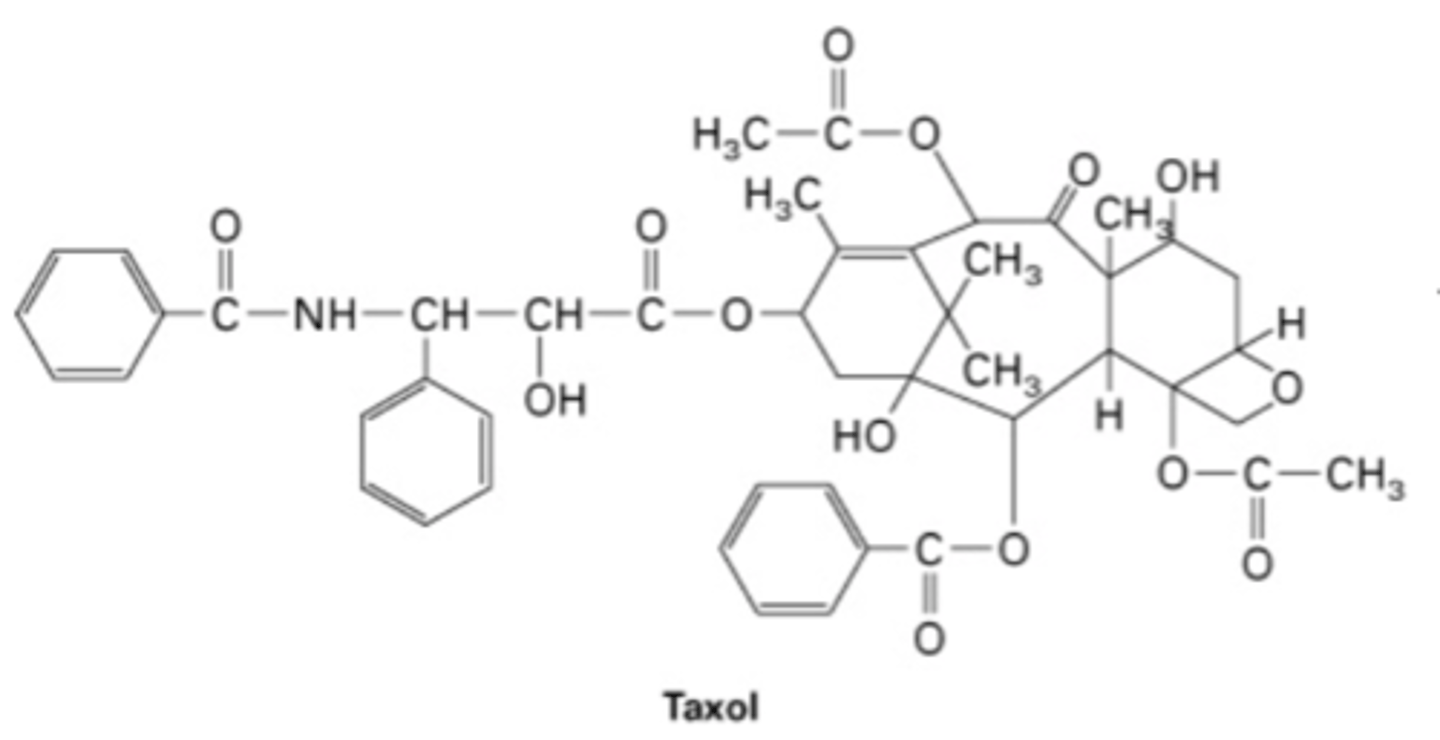
colchicine and taxol
drugs that disrupt microtubule dynamics
- bind between α and β tubulin on dimers
- high concentration: causes depolymerization
- low concentration: stabilizes
colchicine
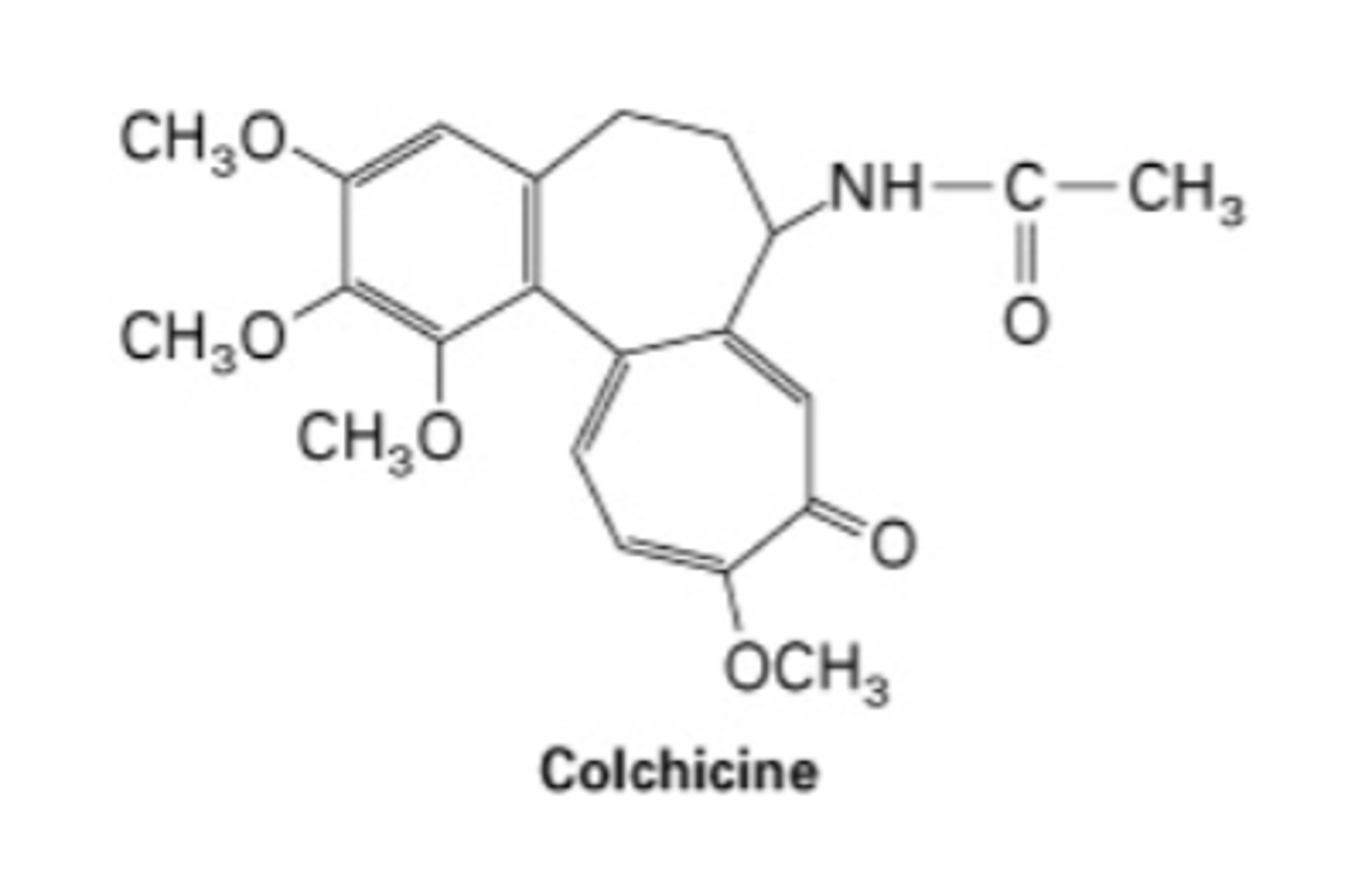
- binds to side of tubules
- stabilizes
- used in chemotherapy, messes up cell divison
taxol
1. microtubules-associated proteins (MAPs) (stability)
2. proteins that regulate (+) end polymerization/connect MT to other structures (+TIPS (plus-end trafficking proteins)
3. proteins that regulate (+) end depolymerization
4. motor proteins, intraflagellar transport, ciliopathies
proteins associated with microtubules
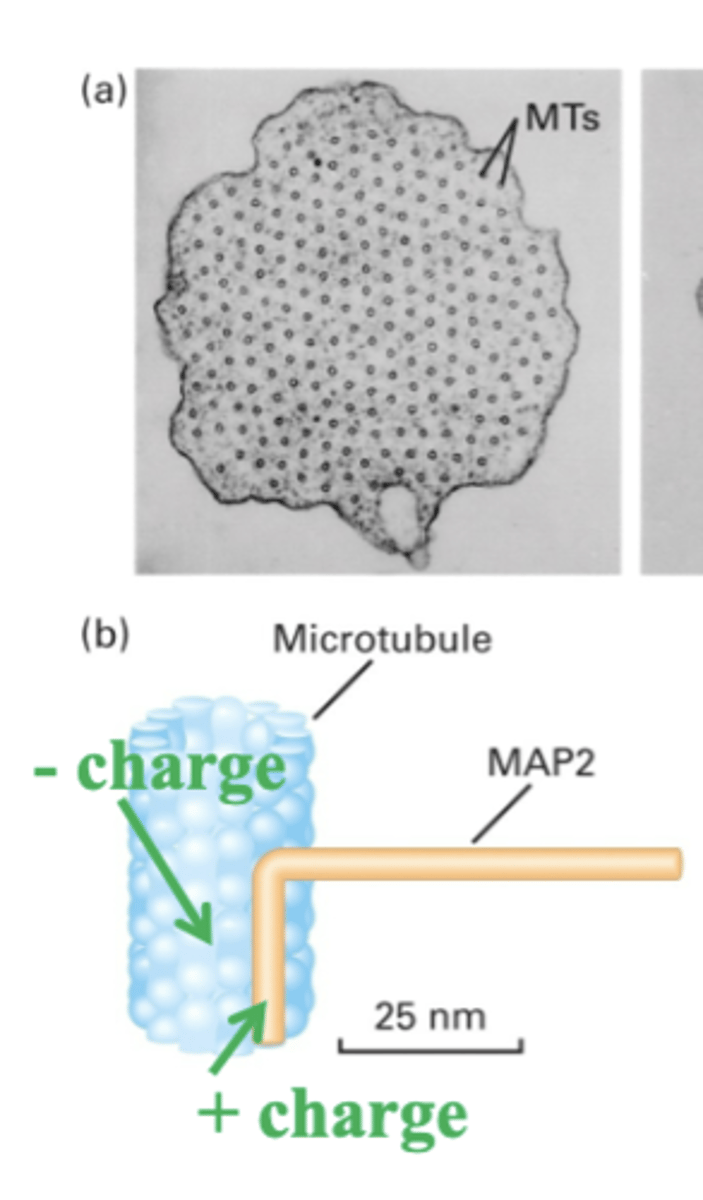
stabilize MTs. interact with side of MT => different MAPs have different arm lengths
Microtubules associated proteins (MAPs)
MAP with a long arm, has a (+) charge with binds to the (-) charge of MT
MAP2
MAP with short arm
Tau
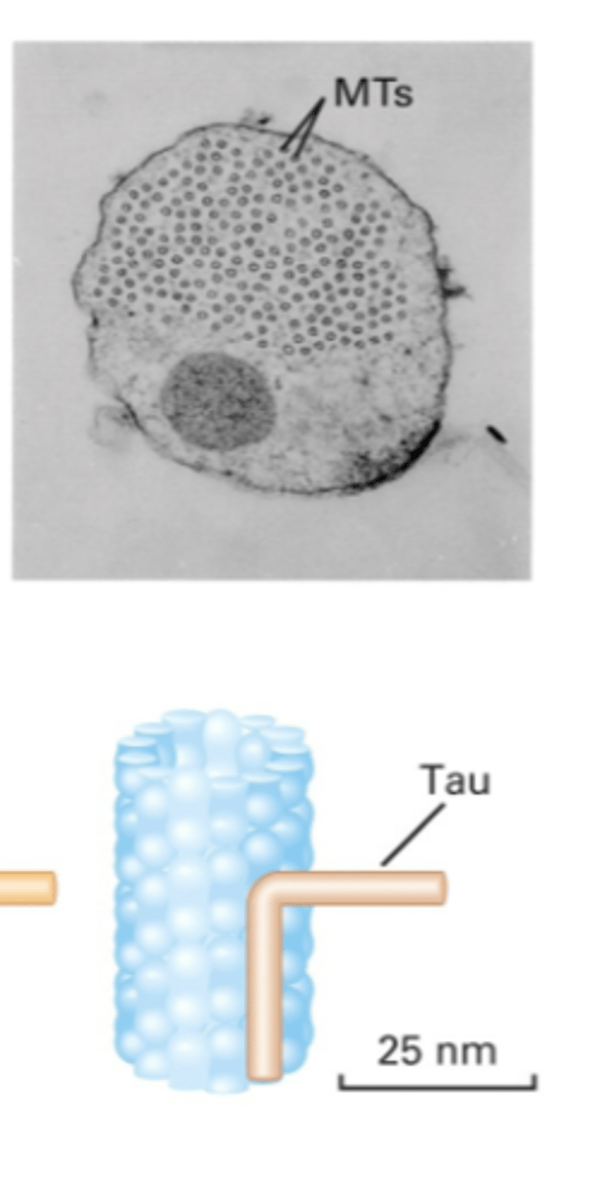
different organization
different arm lengths of MAPs cause?
plus-end trafficking proteins
regulate polymerization at the (+) end, e.g. EB1 and EB3
+TIPs
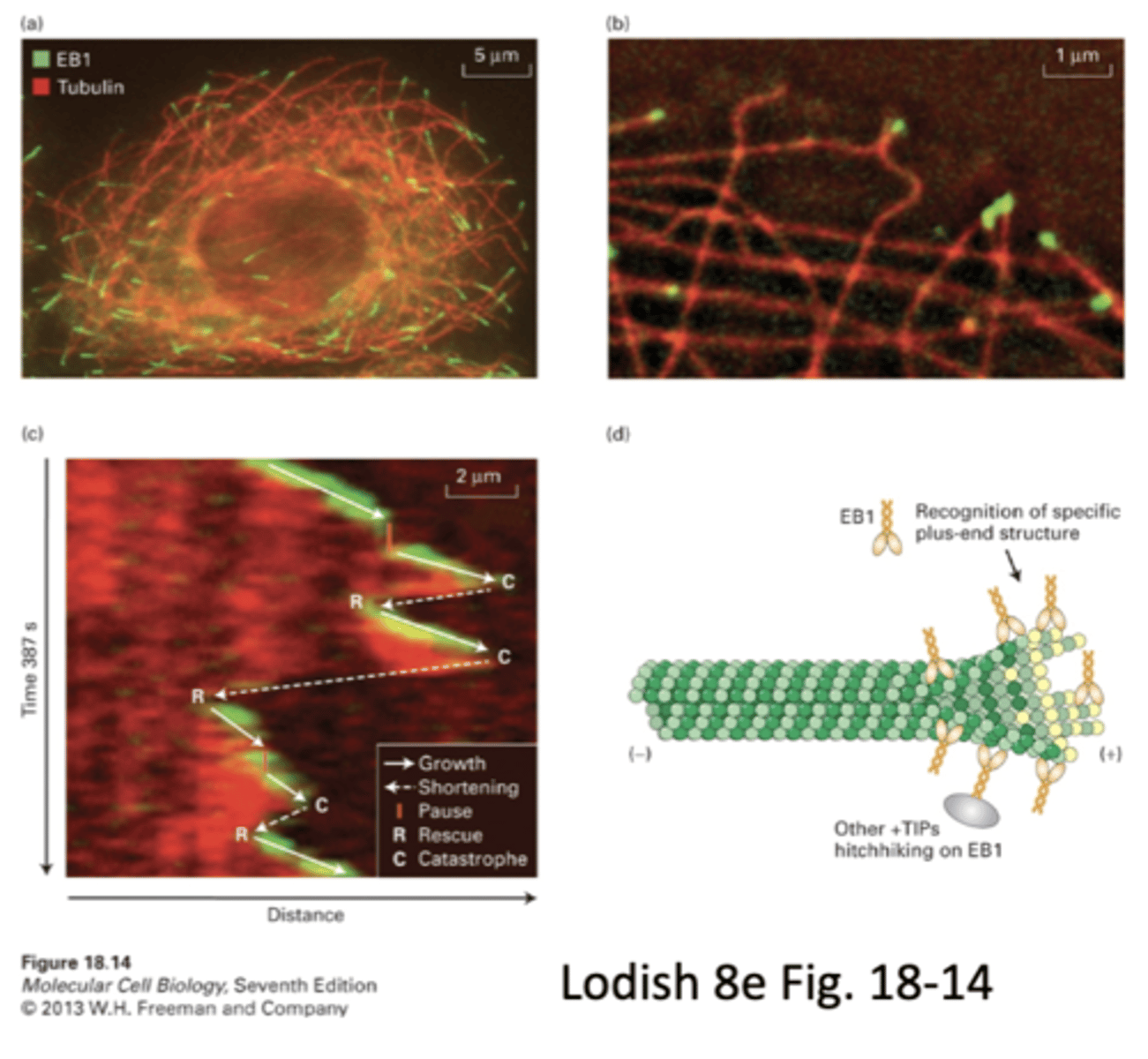
1. promote growth
2. prevent disassembly
3. connect MTs to other structures
how do +TIPs work?
stacked images of same MT show that +TIPs associate with MT at the (+) end. can visualize growth and shrinkage
how do +TIP's like EB1 and EB3 recognize/attach to the + end
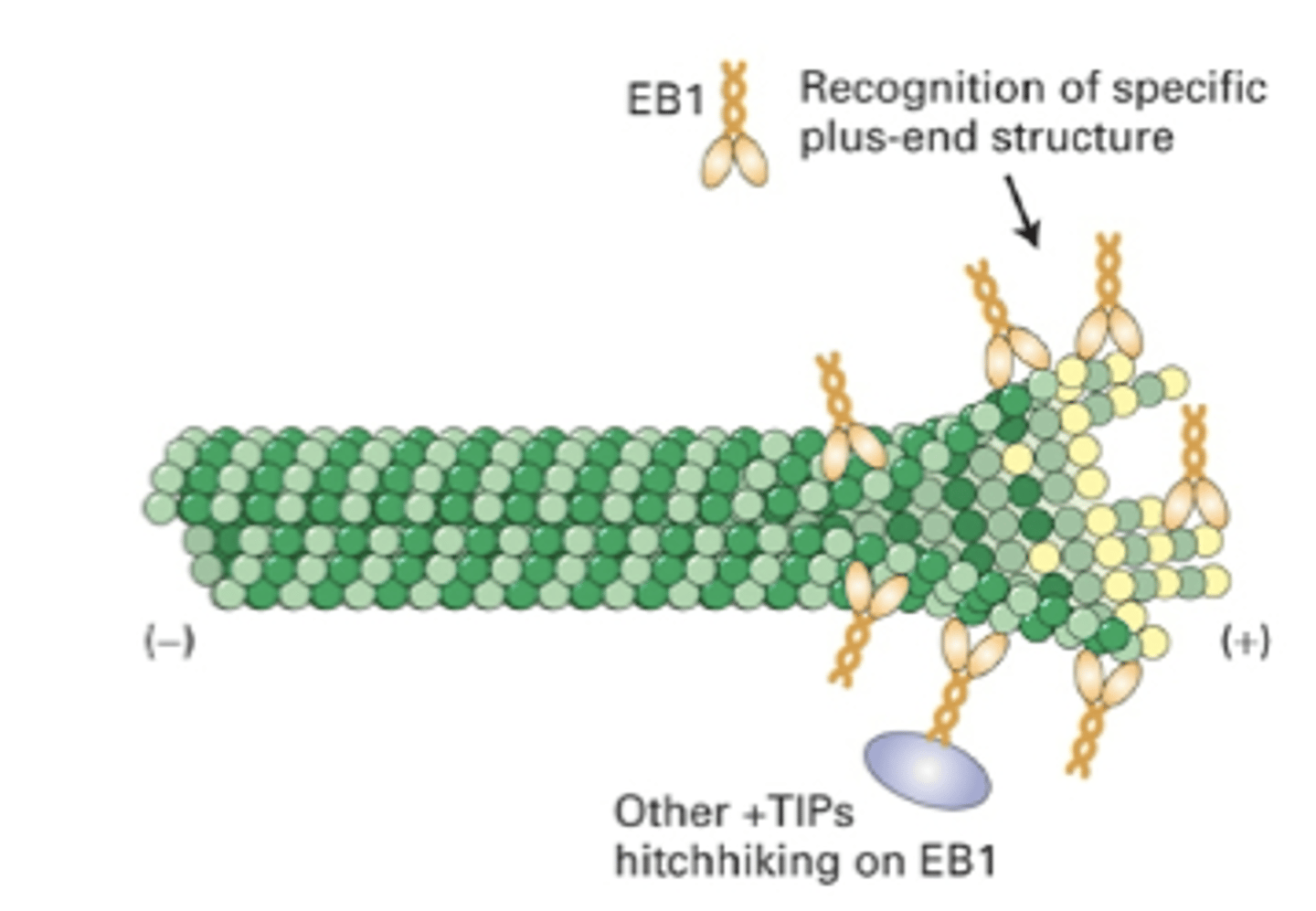
EB1 recognition of specific plus end structure allows it to bind, other +TIPs hitchhike on EB1. could be other proteins that interact as well
proposed mechanism of +TIPS
sometimes rapid reorganization requires loss at both ends, so loss at (+) end occurs too
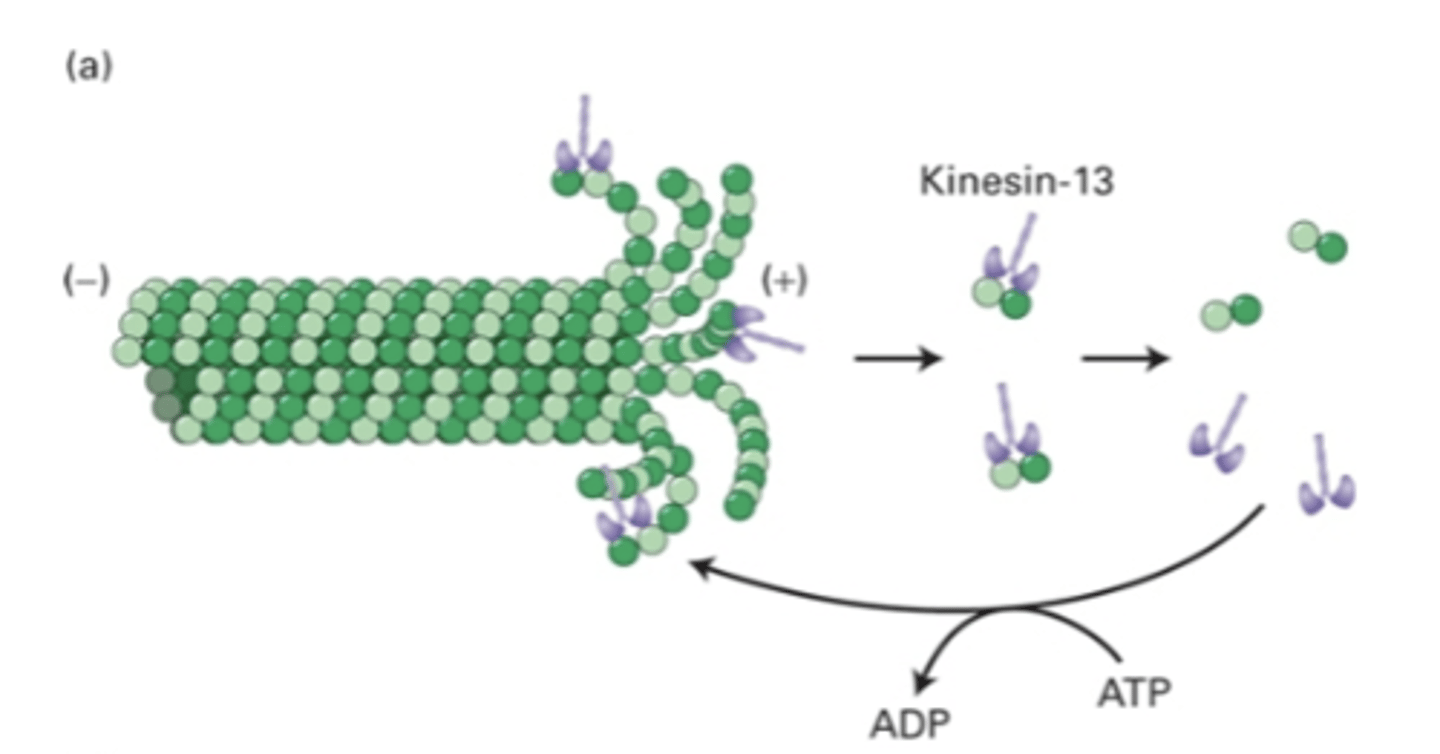
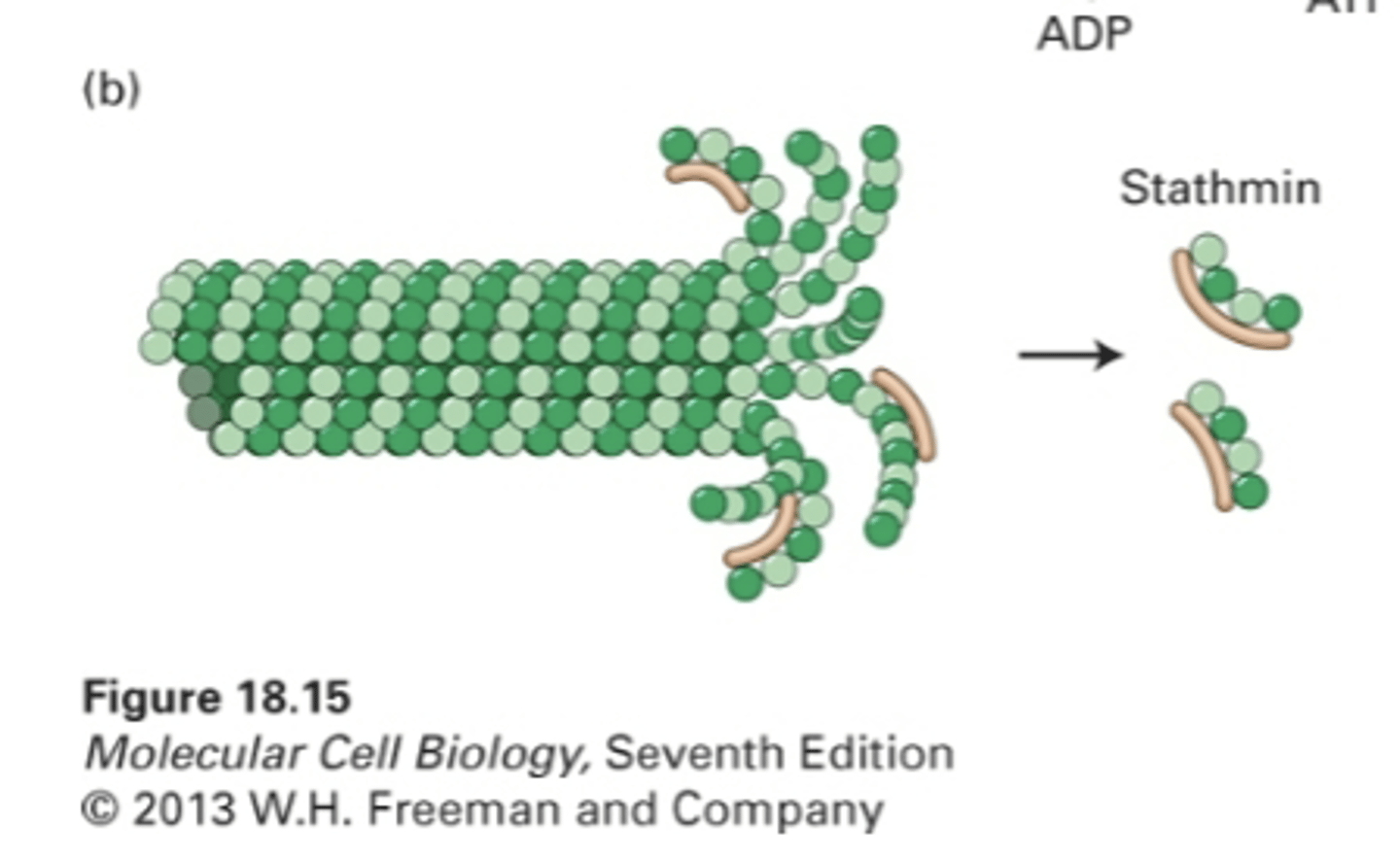
proteins: kinesin-13 and stathmin
proteins that regulate microtubule disassembly at (+) end
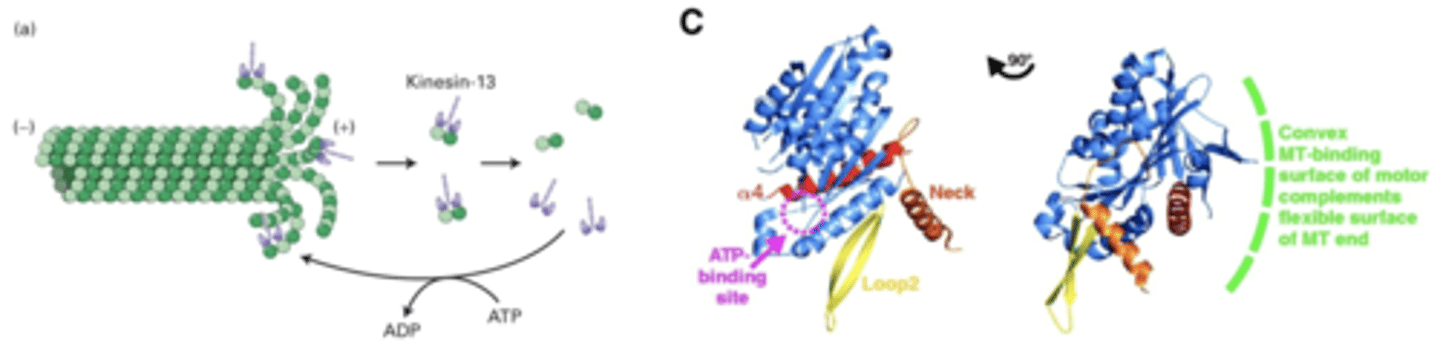
binds to (+) end, uses ATP hydrolysis to bend MT. destabilizes MT so they fall off more quickly
end disassembly, bend MT to promote disassembly
kinesin-13
enhances intrinsic GTP hydrolysis by increasing activity of B4
stathmin
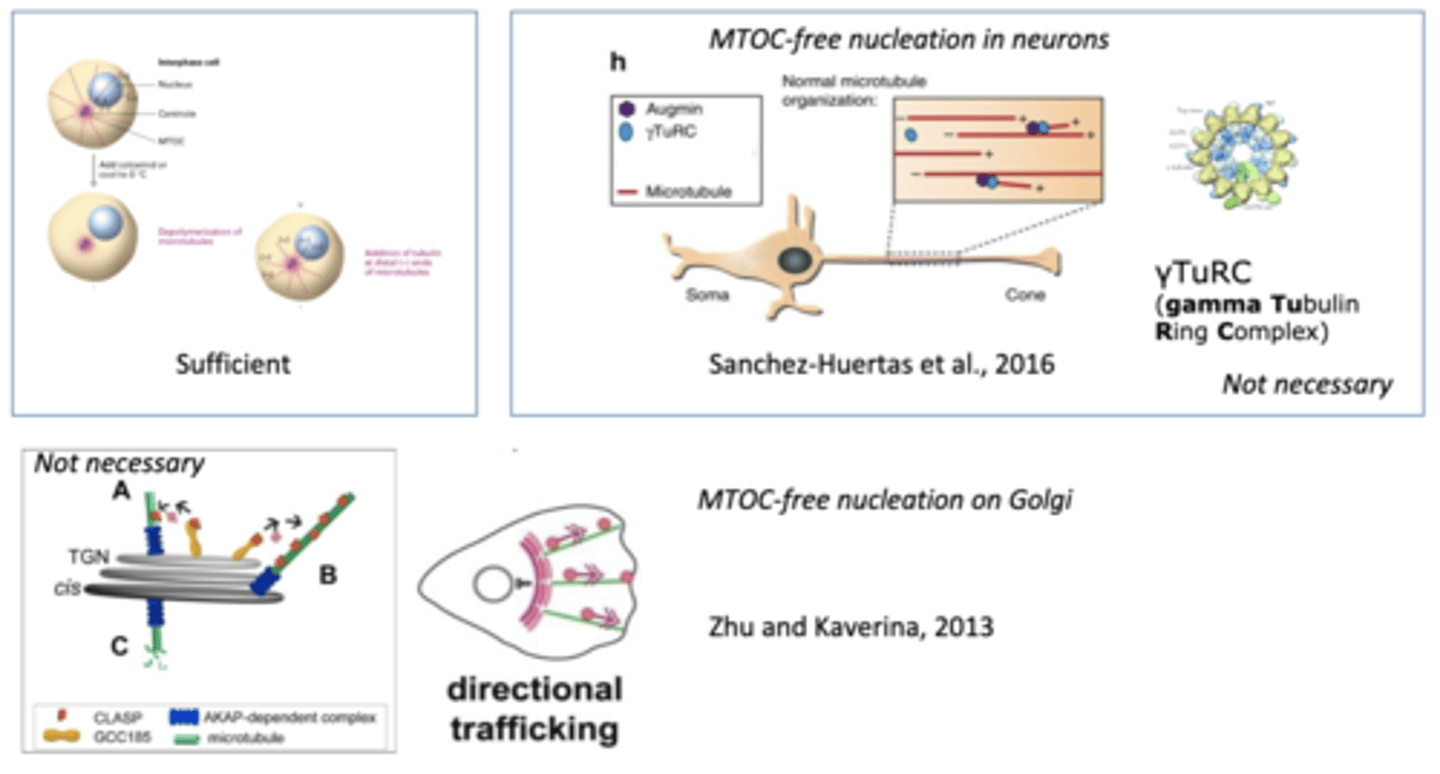
sufficient, necessary
MTOC are ______________ to nucleate MT growth, but they are not _________________
kinesin physically bends microtubules by creating a convex shape, which creates force to bend MT
how does kinesin-13 bend MT?
kinesins (cytosolic) and dyneins (cytosolic + axonemal)
two families of microtubule-based motor proteins
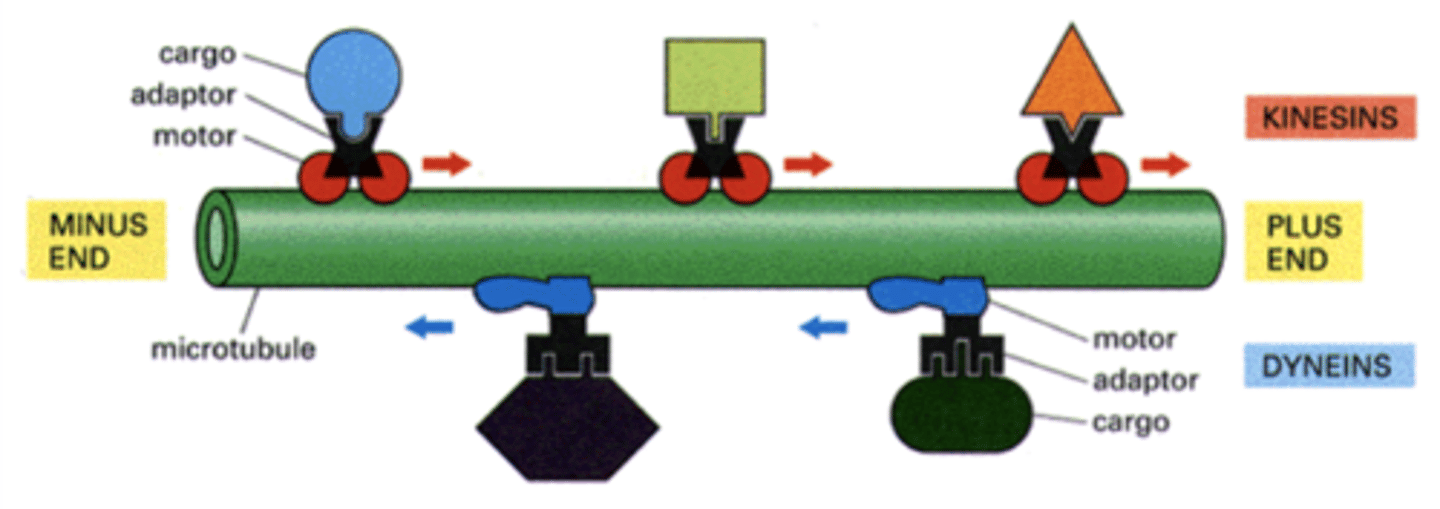
+
Kinesins move towards the _____ end of the microtubule.
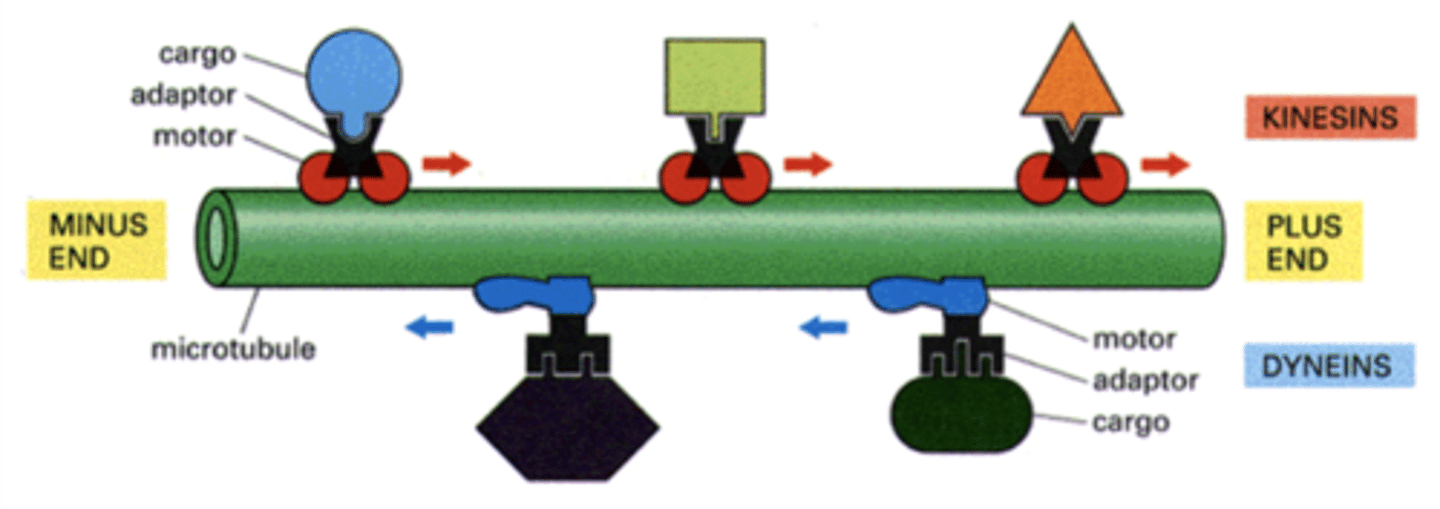
-
dyneins move towards the _____ end of the microtubule.
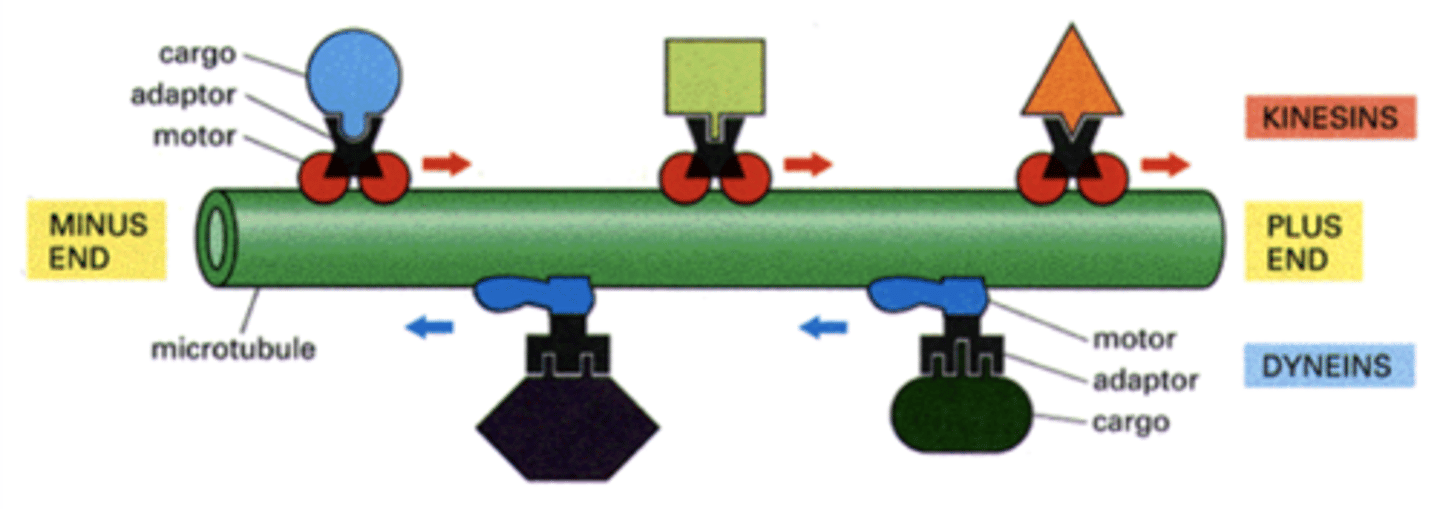
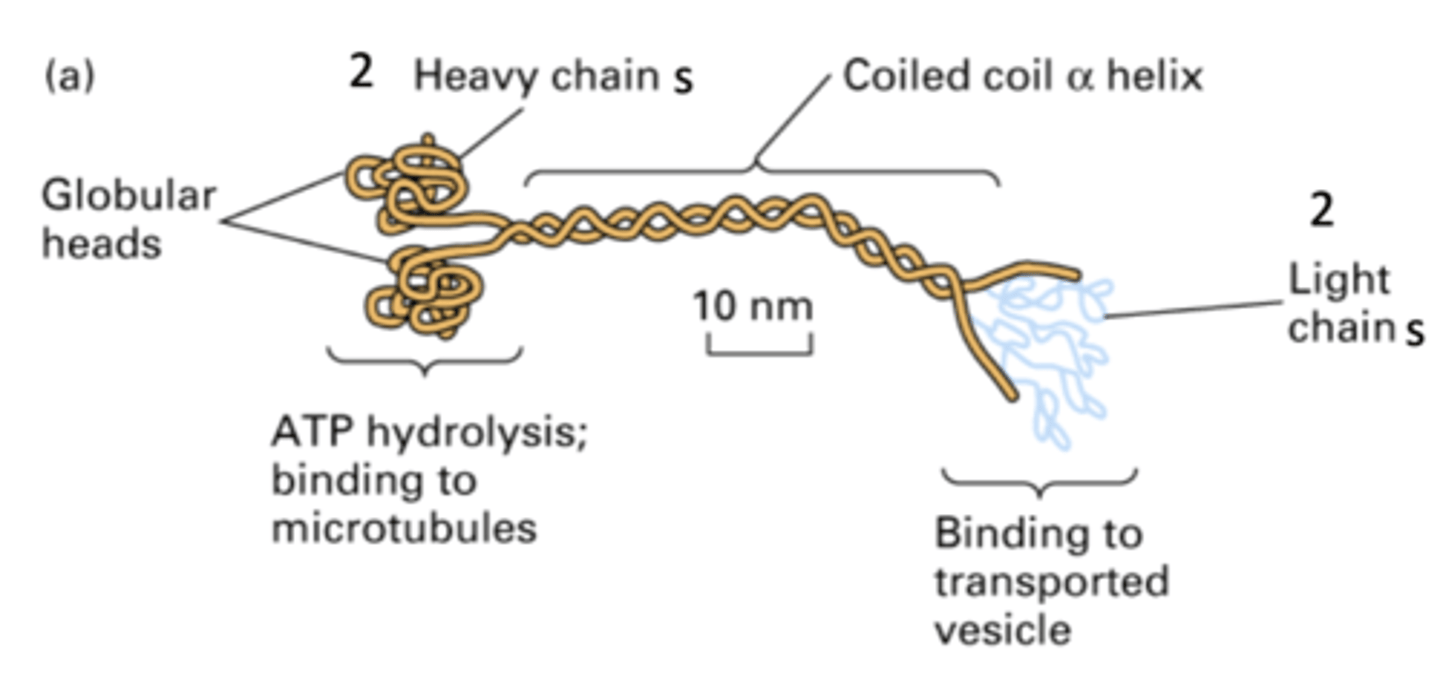
- globular heads (bind to strand)
- 2 heavy chains where ATP hydrolysis occurs, binding to MT
- coiled-coil α helix
- 2 light chains that bind to transported vesicles, help with cargo and motor connection
EXACTLY LIKE MYOSIN
cytosolic kinesin structure
organelle transport
hand-over-hand stepping
Kinesin-1 (conventional) and Kinesin-2 (heterotrimeric)
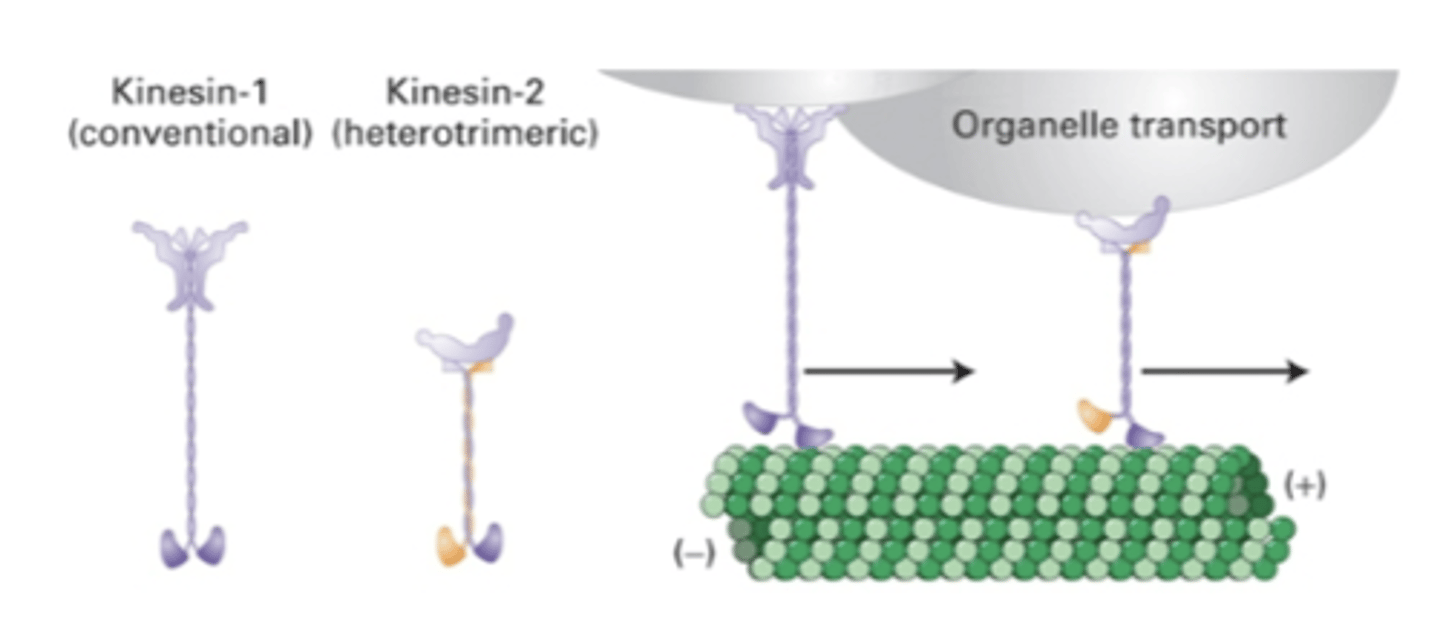
globular heads at both ends
important in organelle organization
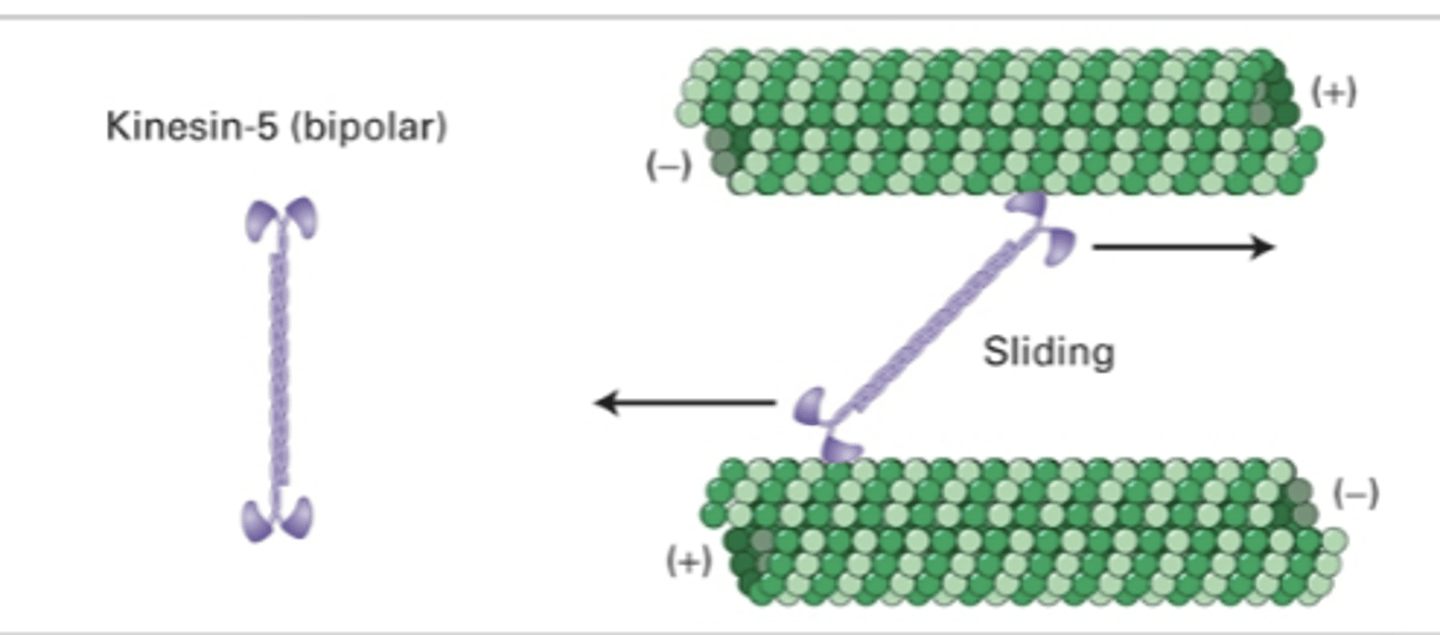
sliding (slide MT in relation to each other)
kinesin-5 (bipolar)
hydrolysis of ATP into ADP promotes stepping mechanism
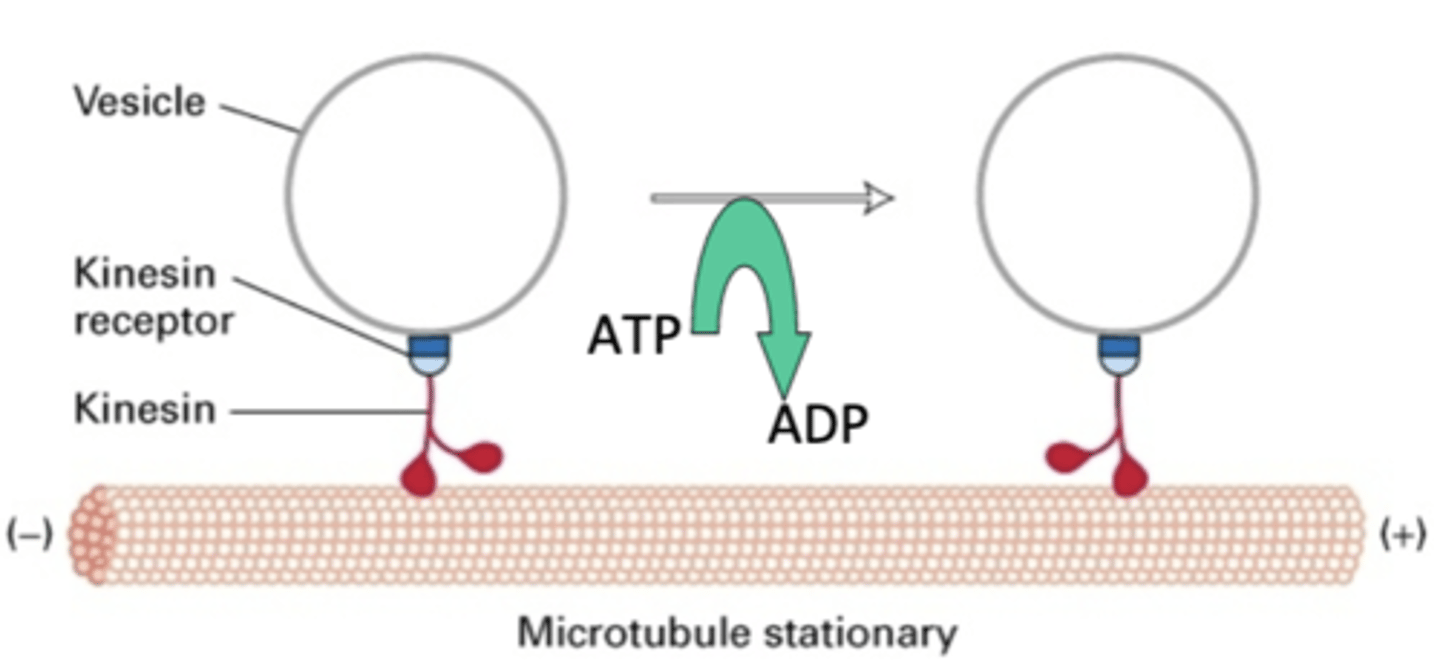
transport of vesicles by cytosolic kinesins
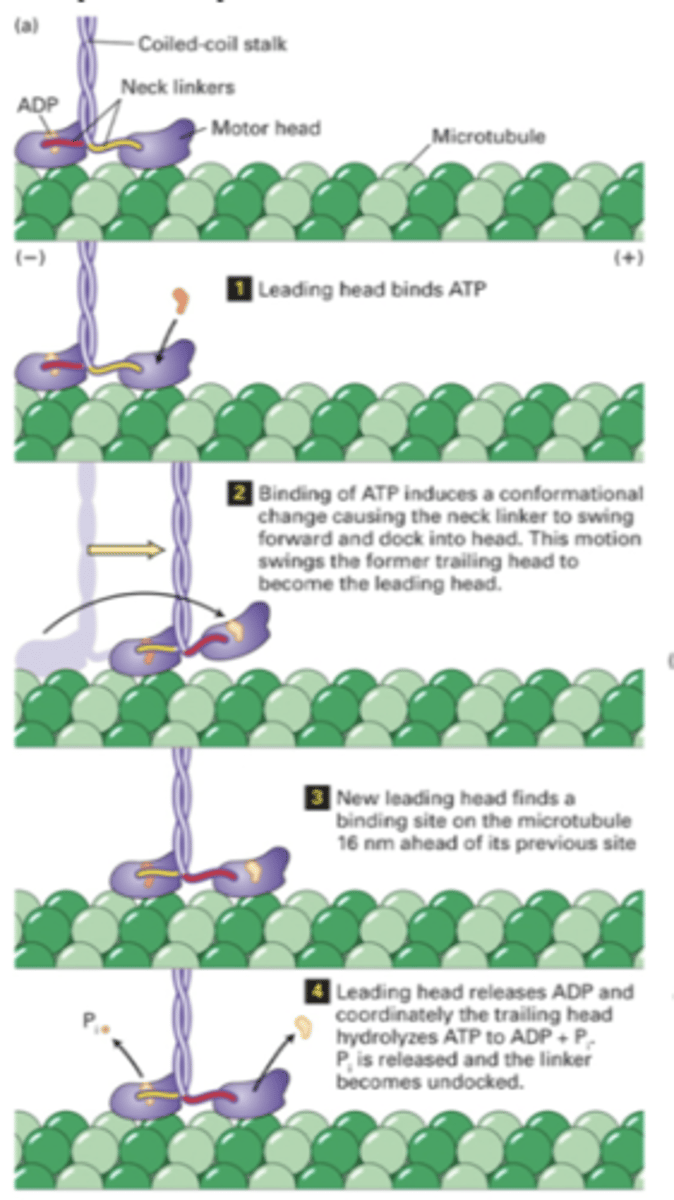
1. leading heads bind ATP
2. lifts off and swings trailing head forward
3. new leading head binds
4. hydrolysis of ATP of old leading end, ATP binding at new leading end
(see image)
motor activity of cytosolic kinesins
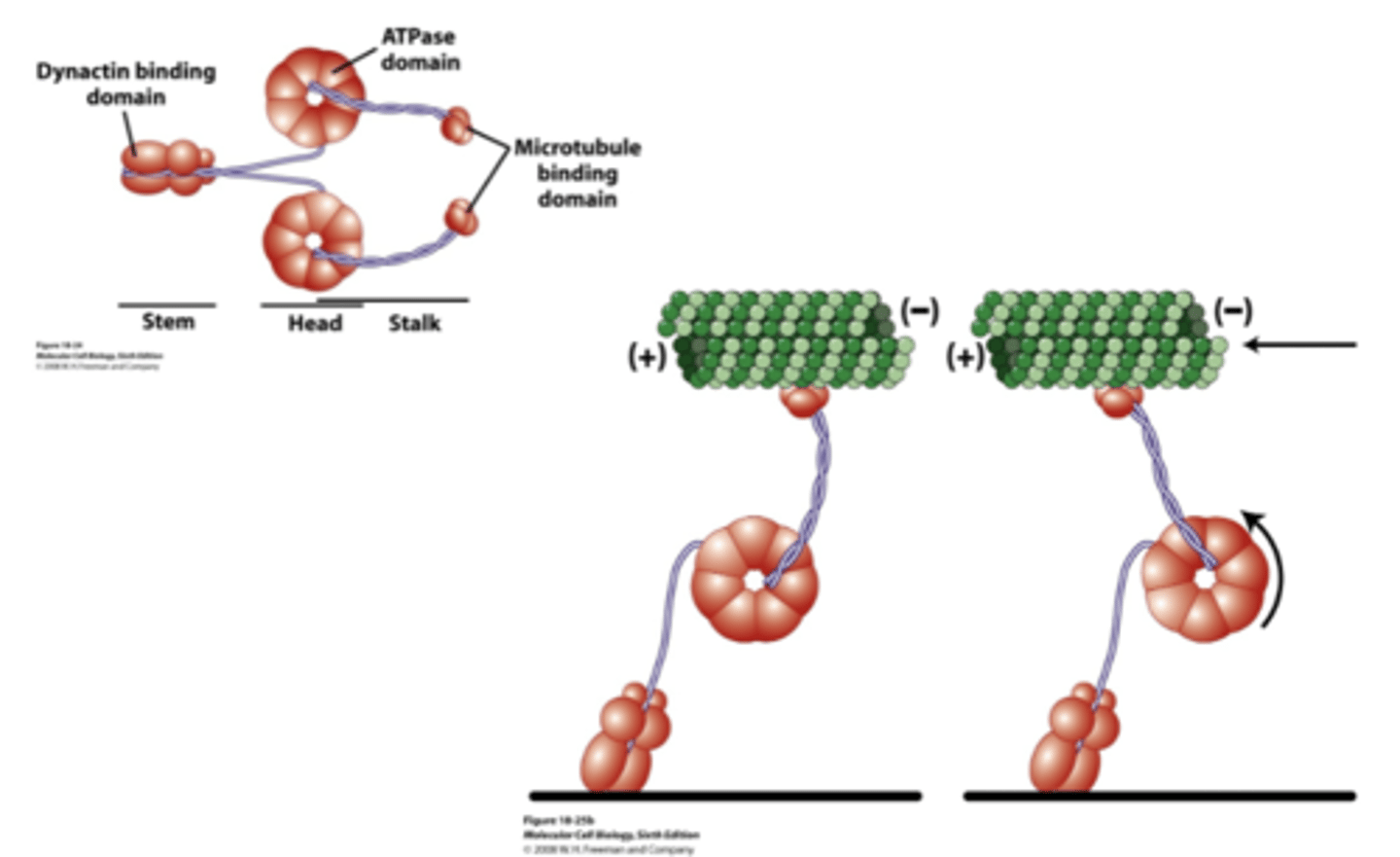
- stem with dynactin binding domain (interacts with cargo)
- head with ATPase domain
- stalk with mictotubule binding domain
structure of cytosolic dynein
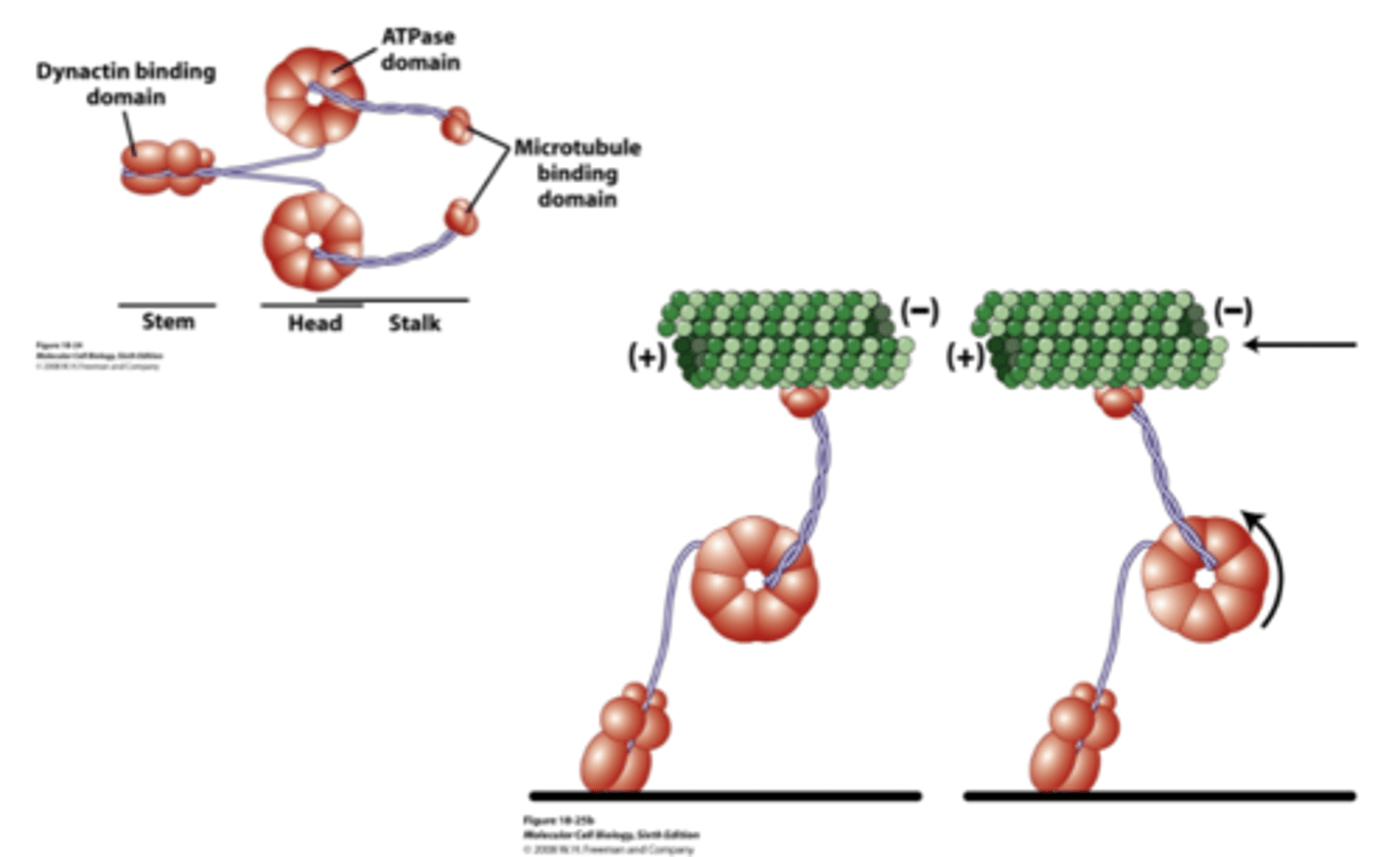
move towards (-) end of ginormous proteins (4000 AA0
rotates, which moves MT-binding domain towards (-) end
motor activity of cytosolic dynein
kinesin, dynein
_______________ move cargo away from MTOC while ______________ move cargo towards MTOC
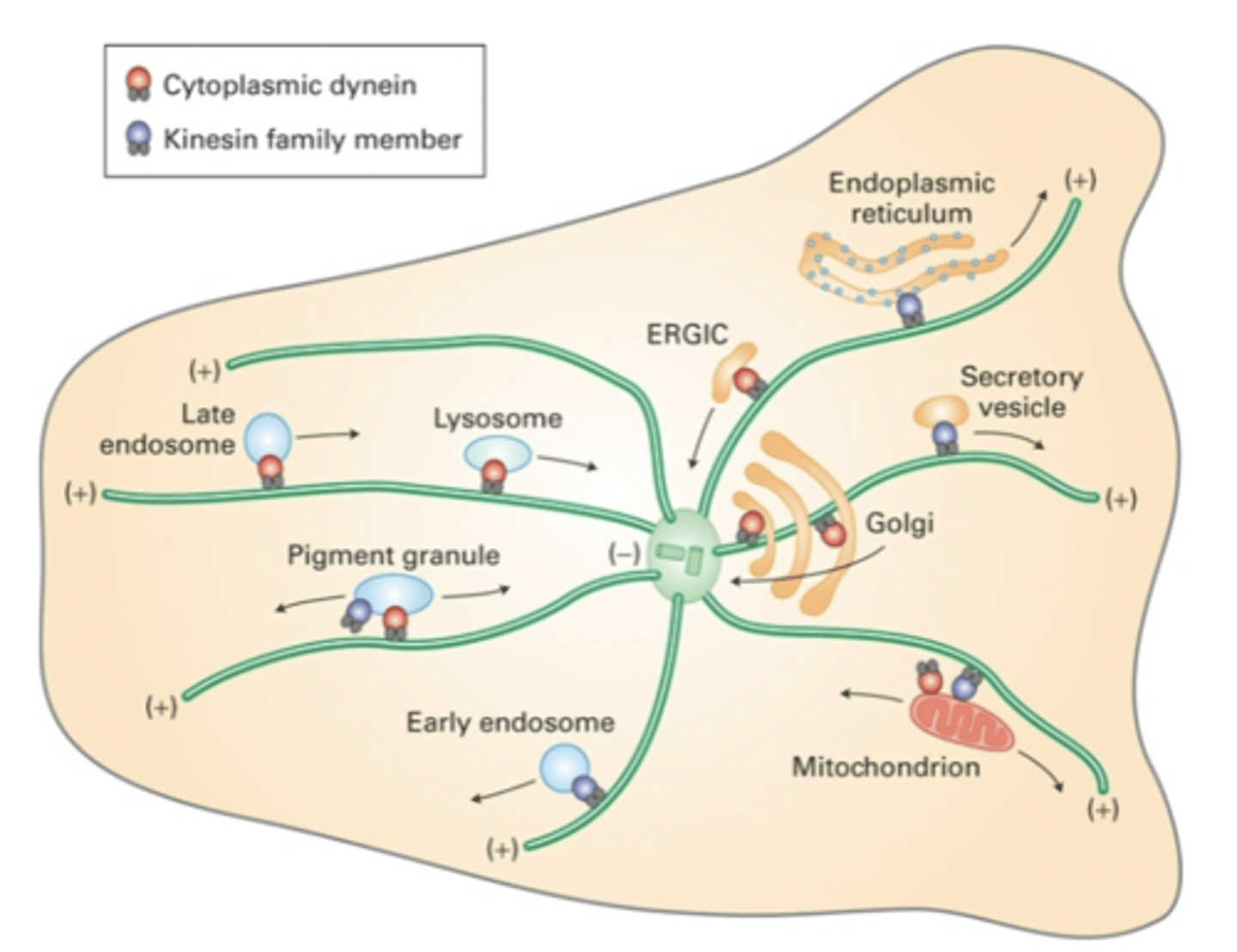
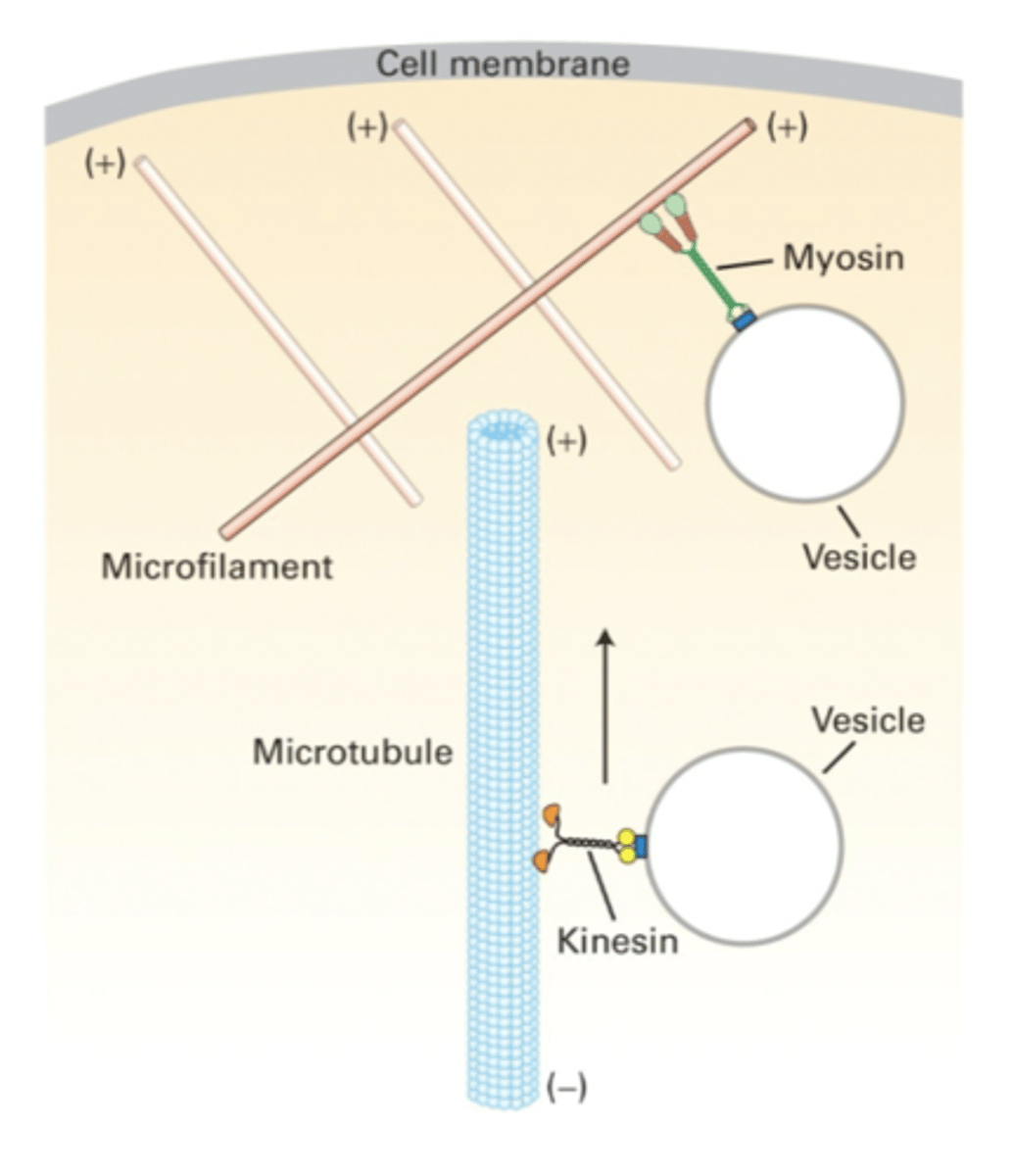
actin, microtuble
cooperation between ________-based and _______-based motor proteins allows the movement of vesicles through the cell
- 1/2 per cell
- locomotion (sperm, single-cell eukaryotes)
flagella
- many per cell (all around cell)
- locomotion (single cell eukaryotes)
- moving particles or fluid over cell surface (tracheal cells)
cilia
"9+2" arrangement = 9 doublets (A and B subunit) and 2 singlets
structure of axonemes of cilia or flagella
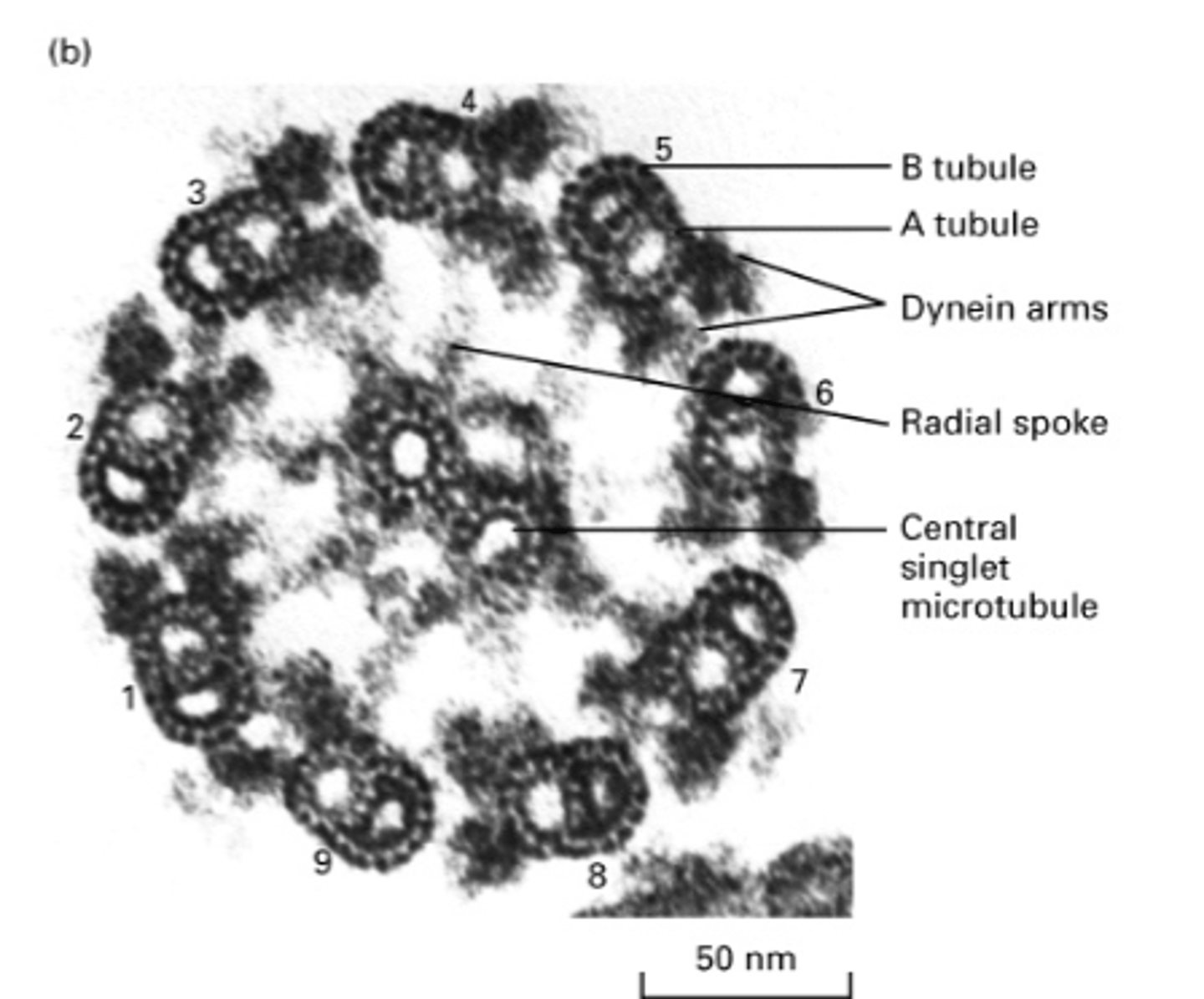
outer dynein arm
What is this?
what is this?
outer dynein arm
what is this?
inner dynein arm
what is this?
plasma membrane
what is this?
nexin
what is this?
bridge connecting central singlets
what is this?
central pair of singlet MT
what is this?
inner sheath
what is this?
B tubule
what is this?
A tubule
what is this?
doublet MT
what is this?
spokehead
what is this?
radial spoke
A tubule has how many protofilaments?
13
B tubule has how many protofilaments?
10
A protein that links microtubule doublets to each other in the axoneme, important in flagellal beating
nexin
-
the inner and outer dynein arms move toward the ___ end
radial spoke
inner sheath
what parts of the axoneme are important in structural integrity?
1 base
3 heads
large
structure of axonemal dynein
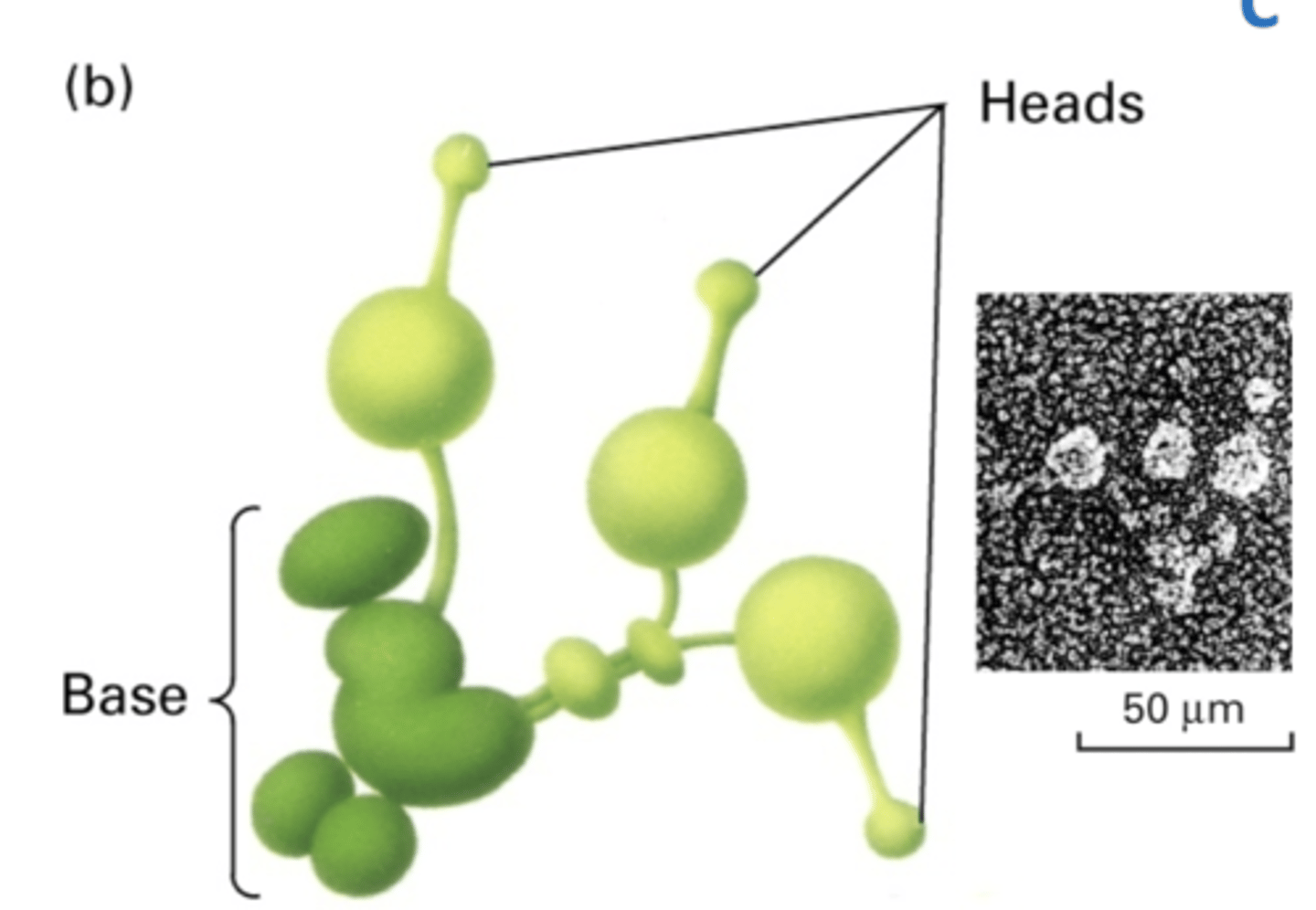
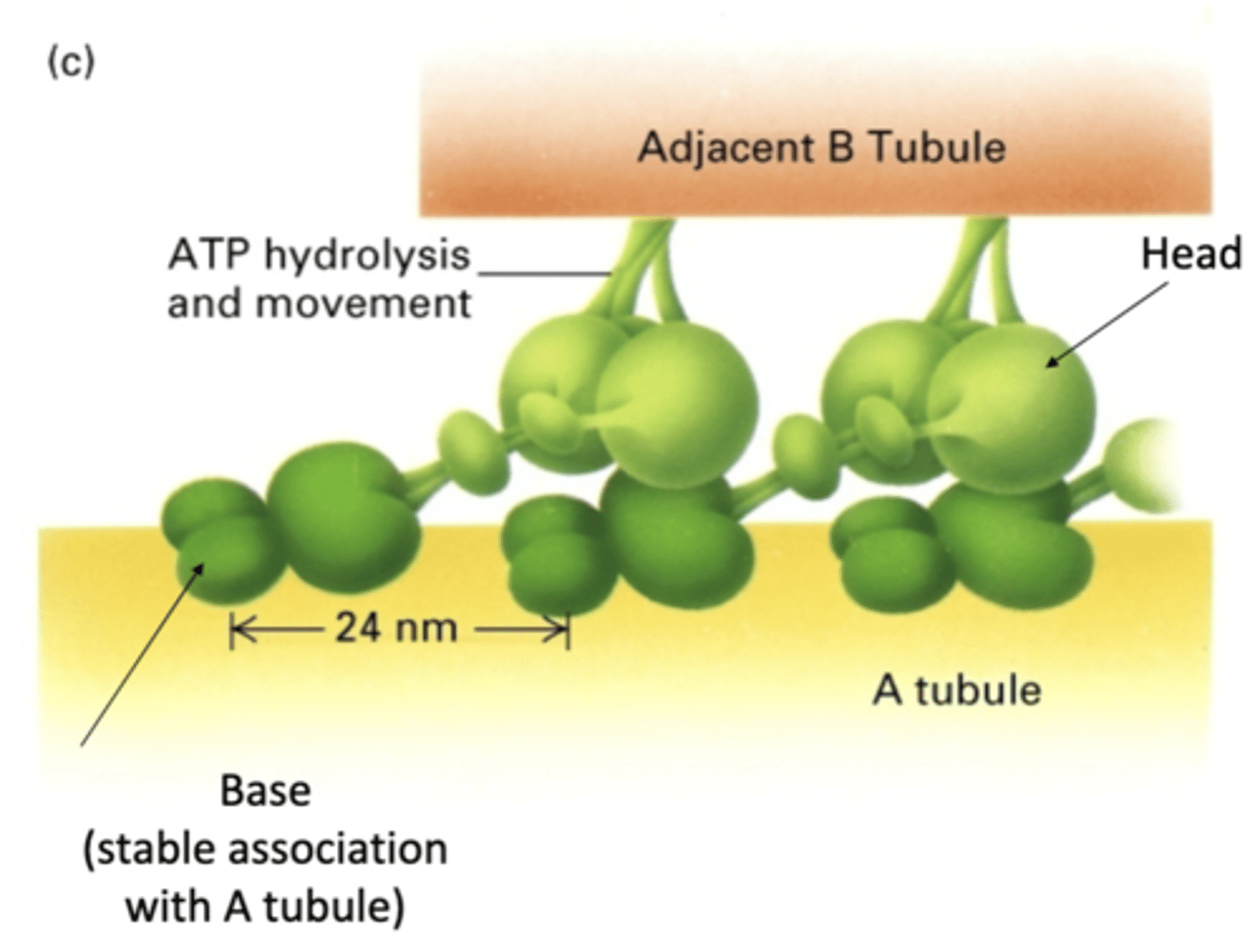
base has stable association with A tubule
head has transient interactions with B tubule using ATP hydrolysis
base binds with A tubule while head walks along neighboring B tubule (not same A and B pair)
how does axonemal dynein interact with A and B tubules?
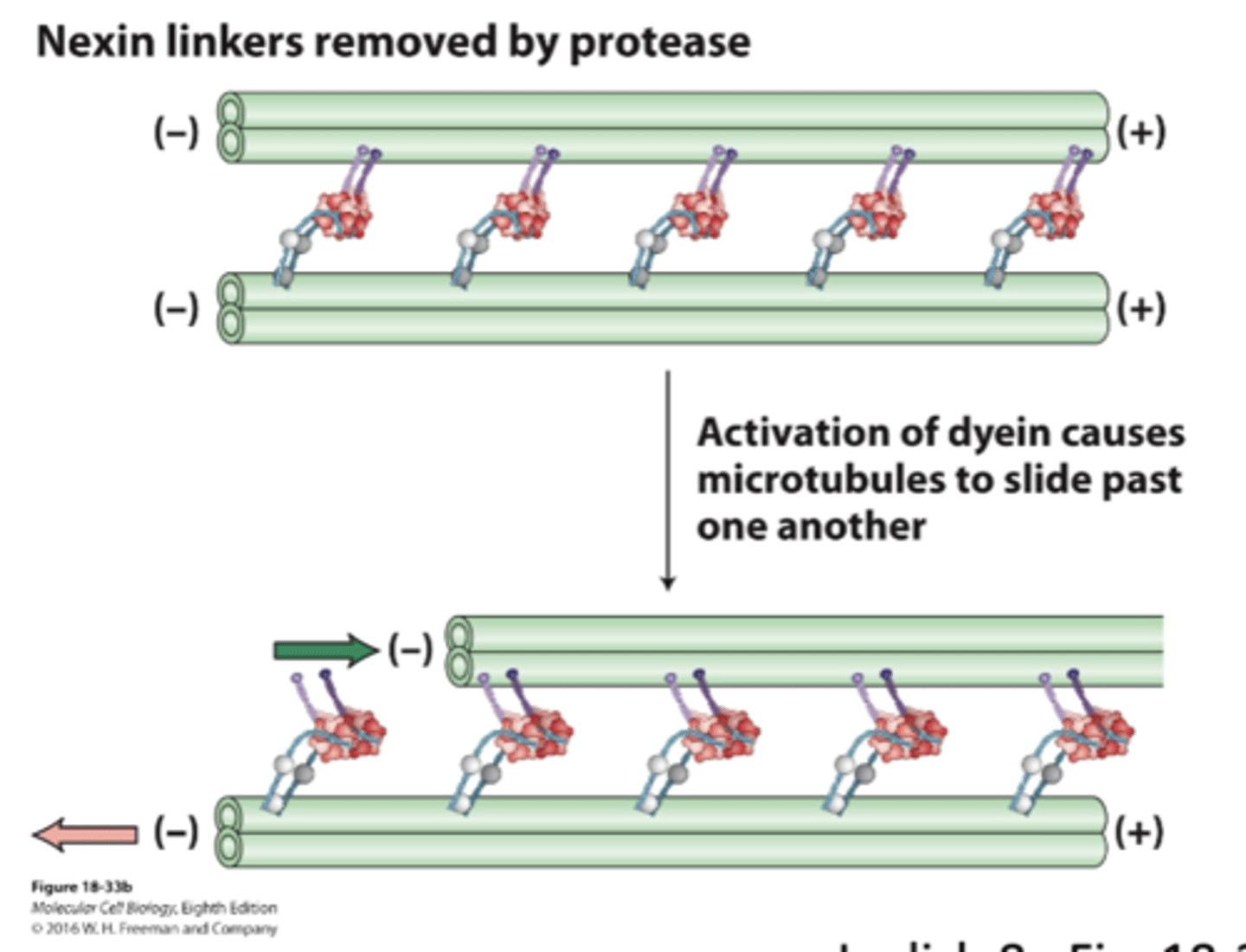
nexin connects tubulin pairs, so when the tubule tries to move (because of dynein activation) it can't. results in bending, which is progated throughout flagella and creates a wave
dynein sliding of axonemal microtubule doublets
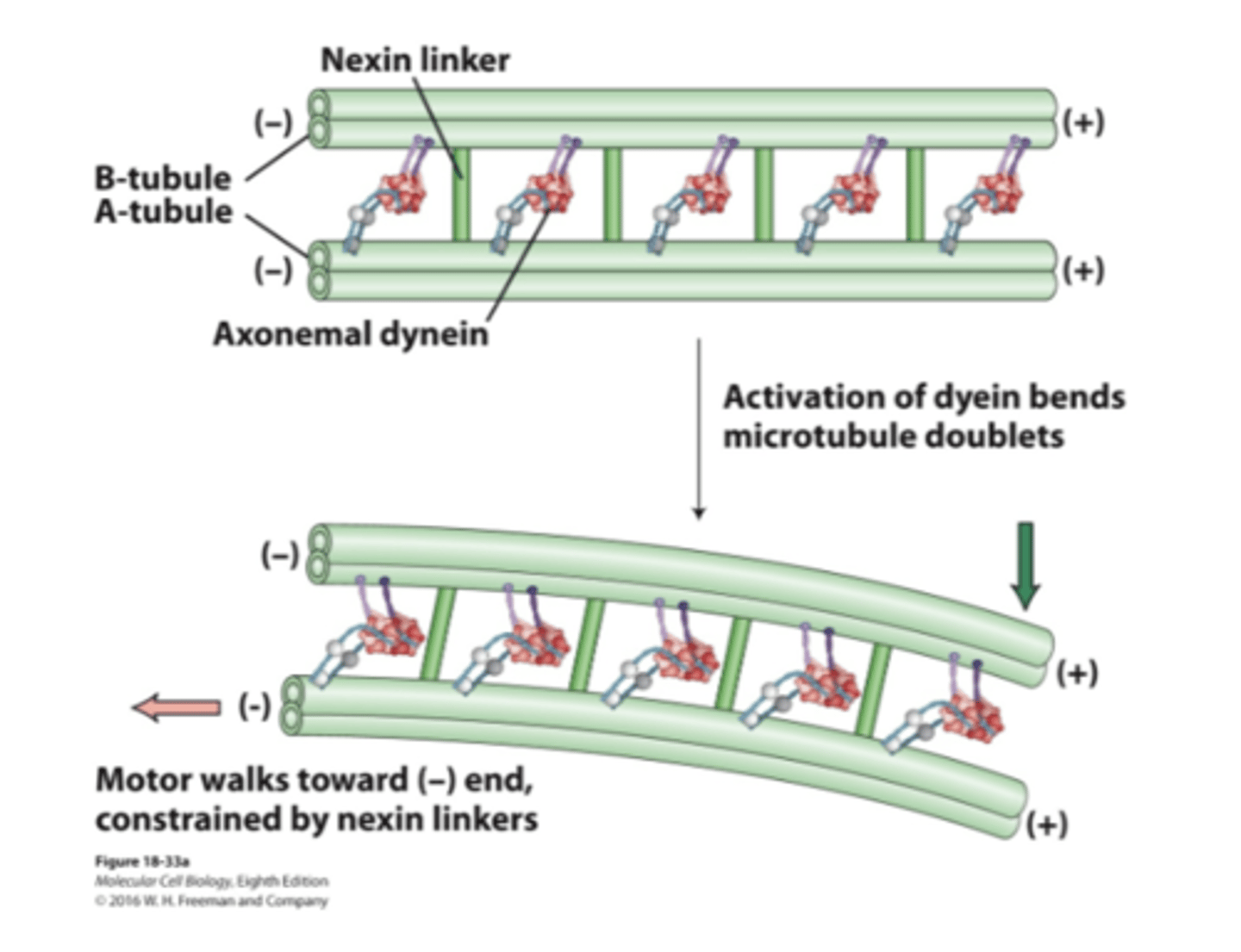
no nexin => no sliding
activation of dynein causes MT to slide past one another as they walk towards (-) end. think of it as people "dragging" tubule
dynein sliding of axonemal microtubule doublets when nexin linkers removed by protease