5.6- Redox Titrations and Electrode Potentials
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
variable oxidation states
when one element can have different oxidation states
common in transition metals
redox reactions
a reaction where both oxidation and reduction take place
oxidising agents
a substance that gains electrons to oxidise another substance
reducing agent
a substance that donates electrons to reduce another substance
forming redox equations
Form two ionic half equations. Half equations can be balanced by adding:
e-
H+
OH-
H2O
add the two half equation to make an overall equation. overall equation must have the same number of electrons on each side of the equation so when balancing you can only add:
H+
OH-
H2O
redox titrations
used to determine unknown concentration of a substance
involves transfer of electrons from one species to another resulting in oxidation and reduction
most don’t require an indicator since redox reactions are often self indicating→ colour change between two oxidation states
potassium manganate (VII) titrations
manganate (VII) is oxidising agent→ reduced to Mn2+
iron (II) is reducing agent→ oxidised to Fe3+
reaction mixture must be acidified so xs acid is added to iron (II) ions before reaction begins
choice of acid in permanganate titrations
must not react with manganate ions
dilute sulfuric acid→ does not oxidise under these conditions and does not react with manganate ions
why other acids are not suitable for permanganate titration
HCl→ oxidised to chlorine by manganate ions
HNO4→ is an oxidising agent so may oxidise substance being analysed
CH3COOH→ weak acid, conc. of H+ ions is insufficient
conc. H2SO4→ may oxidise substance being analysed
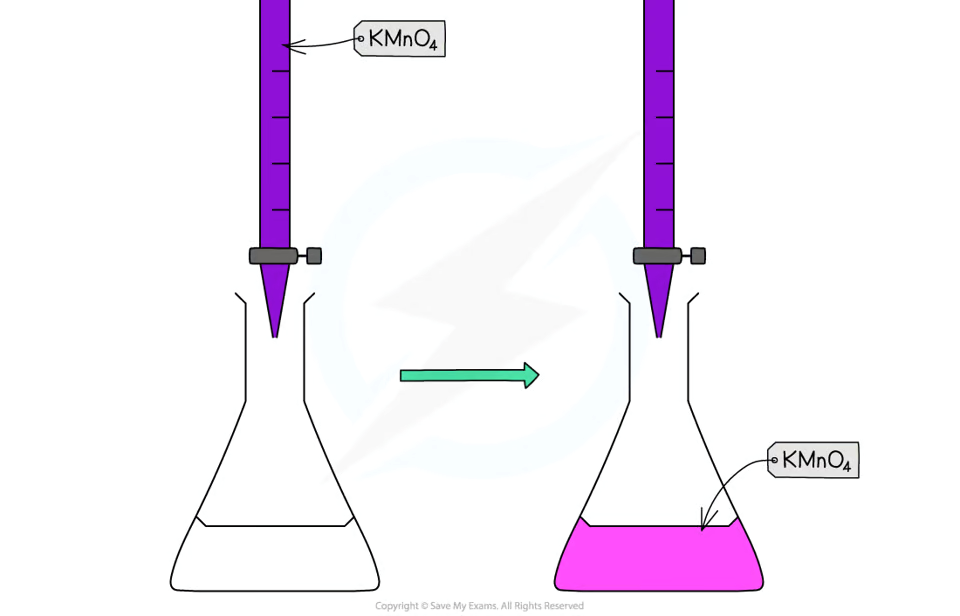
end point in permanganate titration
potassium permanganate acts as its own indicator→ reacts with Fe2+.
potassium permanganate solution is purple
burette must have white numbering to ensure readability of titres.
manganese ions have pale pink colour→ in low concentration so solution looks colourless
when all Fe2+ have reacted, pale pink tinge appears due to excess manganate ions
iodine-thiosulfate titration equation
2S2O32– (aq) + I2 (aq) → 2I–(aq) + S4O62– (aq)
procedure for thiosulfate titrations
Record mass of alloy
dissolve alloy in conc HNO3
dilute acidic Cu2+ solution in a volumetric flask
pipette 25cm3 Cu2+ into conical flask
add Na2CO3 to neutralise xs HNO3:
effervescence
CuCO3 ppt forms
add CH3COOH dropwise until all CuCO3 reacts to form Cu(CH3COO)2:
weak acid used to ensure Cu2+ solution is as close to neutral as possible
titrate liberated iodine with sodium thiosulfate
as colour becomes straw yellow add starch indicator→ clarifies endpoint
END POINT: when blue/black fades, leaving white ppt. in colourless solution
what is thiosulfate titration used for
to determine conc. of oxidising agent→ oxidises iodide ions to iodine molecules
amount of iodine is determined from titration against known quantity of sodium thiosulfate solution
electrochemical cells
convert chemical energy into electrical energy
redox reactions:
oxidation at anode (+ive electrode)
reduction at cathode (-ive electrode)
components of electrochemical cells
external circuit:
wire that allows flow electrons from one electrode to another→ current
salt bridge:
allows ions to transfer between half-cells, maintain charge balance
paper soaked in neutral electrolyte e.g. potassium nitrate
types of half cells
metal/metal ion half cell
ion-ion half cell
metal/metal ion half cell
consists of metal electrode in contact with solution of its own ions
e.g. zinc electrode in solution of Zn2+ ions
metal either oxidised to its ions or ions reduced to metal→ depends on metal’s reactivity
ion-ion half cell
consists of two ions of same element in different oxidation states in contact with inert platinum electrode
e.g. Fe2+ and Fe3+
platinum electrode provides inert surface for e- transfer between ions
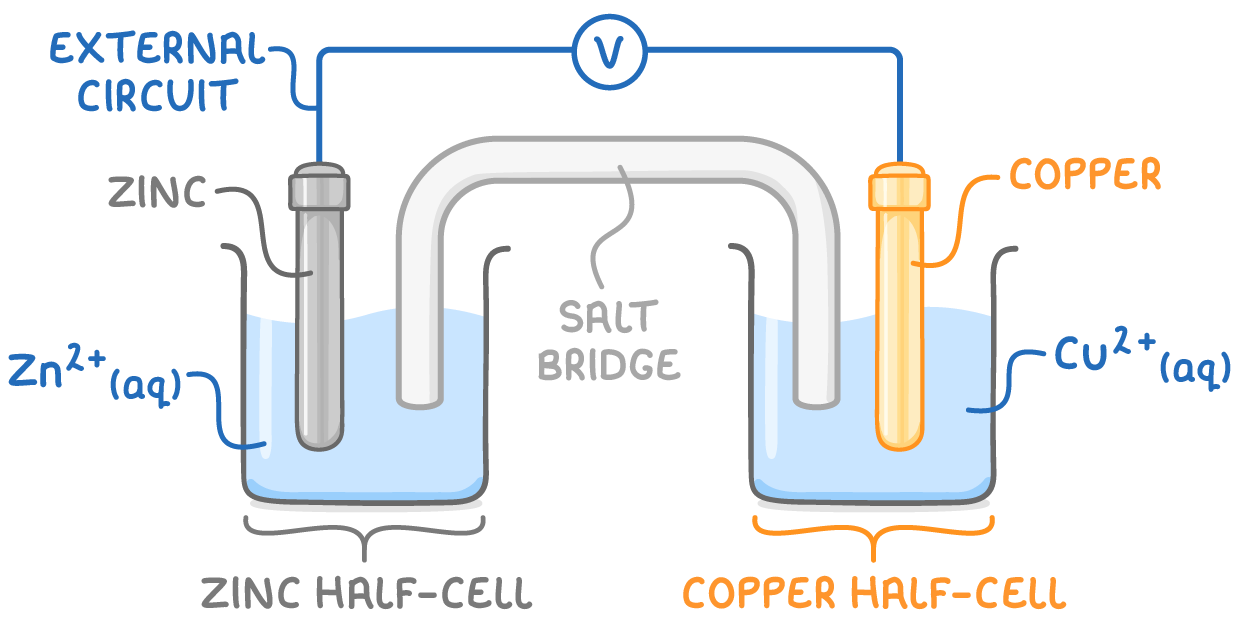
zinc-copper electrochemical cell
at zinc anode, Zn oxidised to Zn2+ ions→ e- released into external circuit
Zn(s) ➔ Zn2+(aq) + 2e-
at copper cathode, Cu2+ ions reduced to Cu→ gain e- from external circuit
Cu2+(aq) + 2e- ➔ Cu(s)
electromotive force
also known as cell potential
e- move from more reactive to less reactive
potential difference measured between half-cells
reversibility of electrode reactions
reaction at each electrode is reversible
direction depends on ease with which metal loses electrons
quantified by electrode potential of each half cell:
more -ive electrode potential= oxidise more readily
less -ive/+ive electrode potential= oxidise less readily
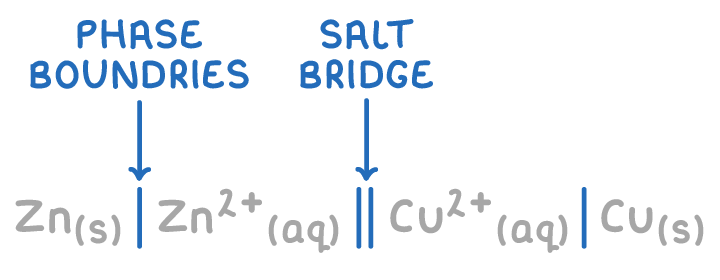
shorthand notation for electrochemical cells
half cell with more negative potential on the left
single vertical line= phase boundary between metal electrode and aqueous ion
double vertical line=salt bridge
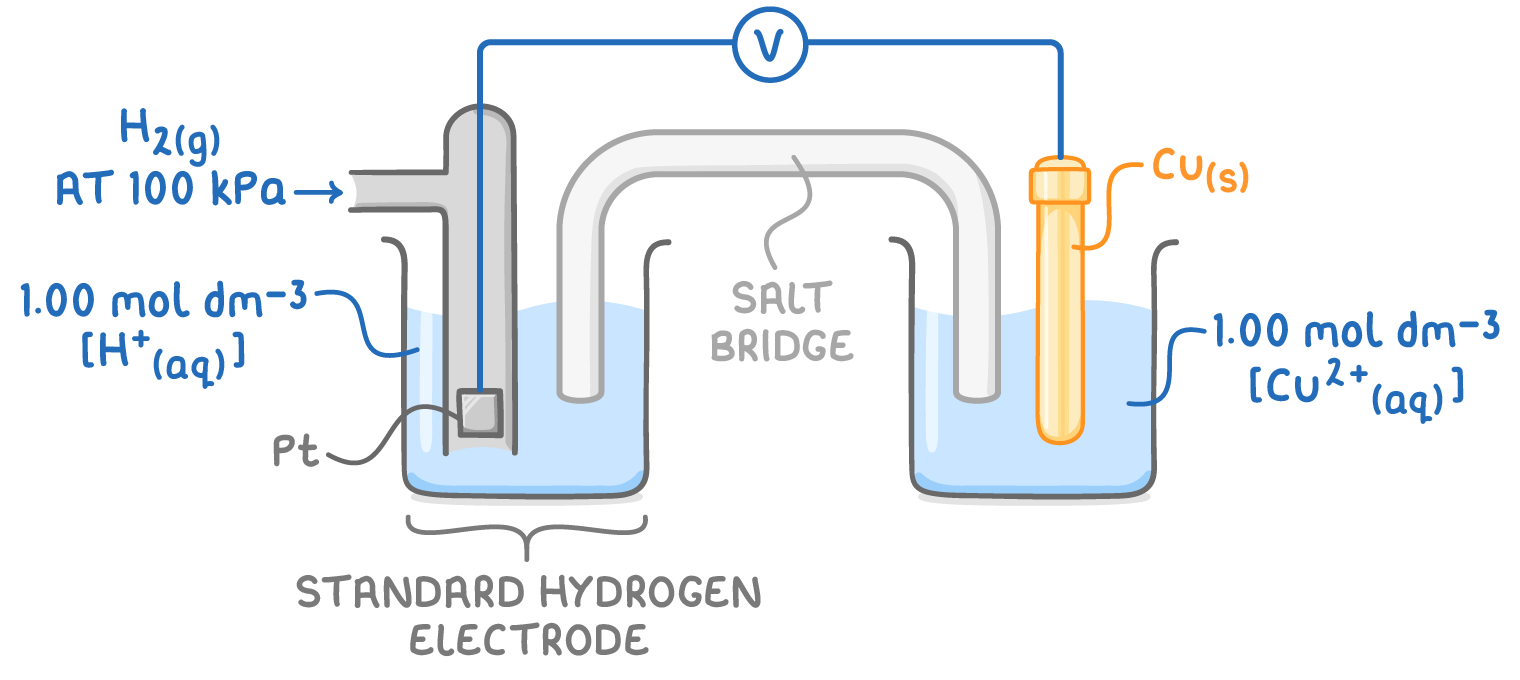
standard hydrogen electrode
used as a universal reference for measuring electrode potentials
features
platinum electrode
immersed in 1.00moldm-3 solution H+
298K
H2 gas at pressure 100kPa bubbling over electrode
hydrogen electrode always positioned on left side of diagrams
the electrochemical series
reduction half equations in order of their standard electrode potentials
most positive E⦵ at bottom→ strongest oxidising agent as it is most readily reduced
most negative E⦵ at top→ strongest reducing agent as it is most readily oxidised
calculating cell potentials from E⦵ values
write reduction half reactions for both species
determine which reaction occurs in oxidation direction (more negative E⦵) and which occurs in reduction direction (more positive E⦵)
combine half equations to give overall redox reaction
calculate E⦵cell
equation for E⦵cell
E⦵cell= E⦵reduced - E⦵oxidised
predicting reaction feasibility using electrode potentials
compare standard reduction potentials of two half equations
more negative E⦵ proceeds in oxidation direction
more positive E⦵ proceeds in reduction direction
if E⦵cell is positive, reaction is feasible→ more positive= more feasible reaction
limitations of using E⦵ for predictions
deviations from standard conditions will affect electrode potentials and alter reaction feasibility
unfavourable reaction kinetics→ reaction predicted to be feasible may proceed very slowly if it has high Ea or slow RoR
standard electrode potentials maybe not accurately predict feasibility for reactions in non-aqueous solvents
energy storage cells (batteries)
contain 2 electrodes with different electrode potentials
potential difference drives cell reaction, allowing electricity generation
fuel cells
can continuously generate electricity as long as fuel and oxygen are provided
in hydrogen fuel cells→ hydrogen and oxygen react to generate electricity
how do hydrogen fuel cells work
at the anode, H2 split into 2H+ and 2e-
platinum catalyst used
protons migrate through electrolyte:
H+ move through polymer electrolyte membrane→ only allows protons to pass through
e- forced to travel through external circuit to get to cathode
electrons flow through external circuit→ generates electrical current
oxygen reduction at cathode:
oxygen combines with H+ and e- to produce water
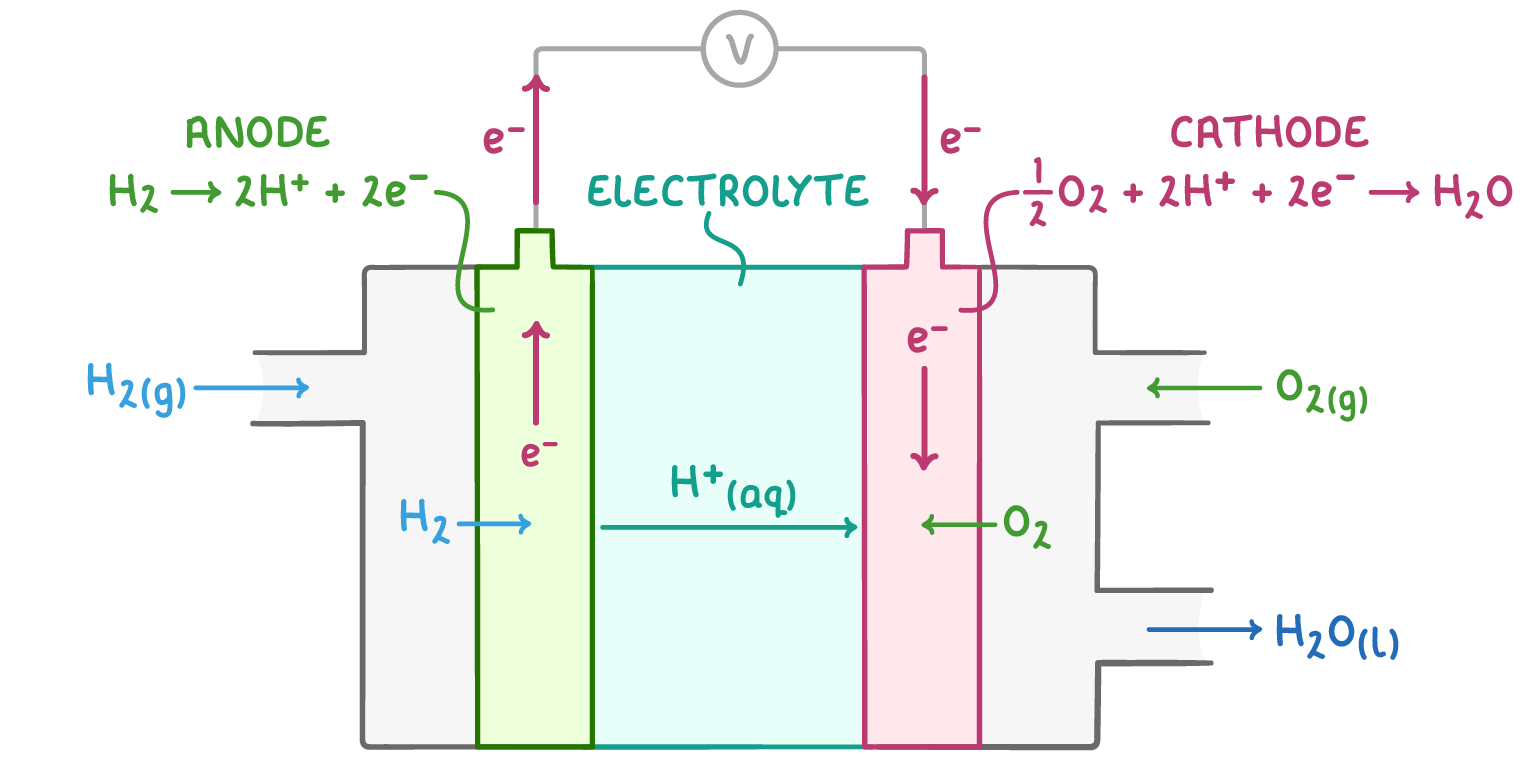
fuel cell reaction equations
negative electrode:
H2 → 2H+(aq) +2e-
positive electrode:
½ O2(g) + 2H+(aq) = 2e- → H2O(l)
overall equation:
2H2(g) + O2(g) → 2H2O(l)
advantages of electrochemical cells compared to combustion engines
more efficient→ convert larger proportion of energy into electrical energy compared to combustion engines (heat)
reduced emission→ produce less pollution e.g. CO2
clean by-products→ e.g. hydrogen fuel cells only produce water as waste product
disadvantages of electrochemical cells
production involves toxic materials that must be disposed of properly at end of cell’s life to avoid environmental damage
chemicals used are often highly flammable