Biochem SI Session 2- Protein Structure II
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
What type of bond is a peptide bond? A disulfide bond? Which AA can have a disulfide bond?
Both are covalent.
Cysteine can form disulfide bonds due to -SH group
Can be intrachain or interchain
Define isoelectric point
pH at which a molecule has no net charge
Found by averaging the 2 pKa values on either side of the zwitterion
Between what parts of the amino acid is a peptide bond formed? Briefly describe a peptide bond
Anhydrous amide (covalent) bond between the α-amino group of one amino acid and the α-carboxylate group of another
Charges on -NH3+ and -COO- disappear
Very hydrophilic and form H-bonds
Stable (take years to break down)
Condensation rxn → H2O is released
What are amino acids in a peptide referred to as?
Amino acid residues
What is of note in terms of the bonds in insulin?
It contains many disulfide bonds, making it a highly disulfide linked peptide (3 total-1 intra, 2 inter)
What is the general length (in residues) of a polypeptide chain?
50-2,000 AA residues
If a mutation replaces a cysteine residue with a serine, how might this affect the protein’s ability to form disulfide bonds? What structural consequence could this have on the protein?
Disulfide bonds would no longer be possible. This would reduce overall stability and could cause misfolding.
The peptide bond has partial double-bond character and is planar. How would protein folding be different if the peptide bond could freely rotate?
This would cause less stability/conformational freedom which would lead to unstable secondary/tertiary structures
Are all peptide bonds trans or cis? What is the exception and why?
Trans due to steric hindrance of R groups
Proline can be found in both since these can be constrained by the ring nature of the R-group
What does the φ angle represent?
The bond between the peptide amide-N and α-C (dihedral angle)
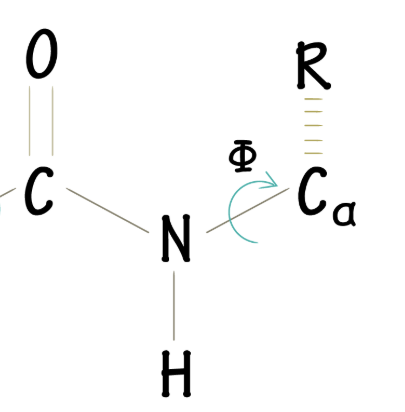
What does the ψ angle represent?
The bond between the α-C and peptide carbonyl carbon (dihedral angle)
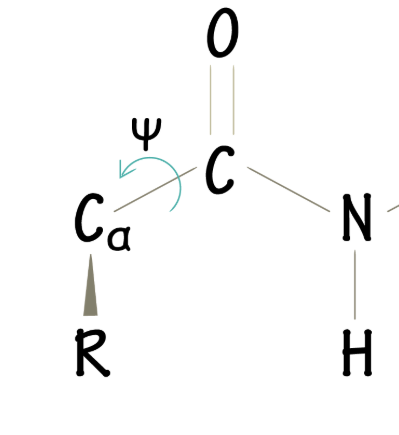
What is a Ramachandran plot?
A representation of all the peptide bond dihedral angles from a single protein represented in a plot
The number of Φ and ψ are highest in two broad “permitted” areas
Most are forbidden due to steric hindrance
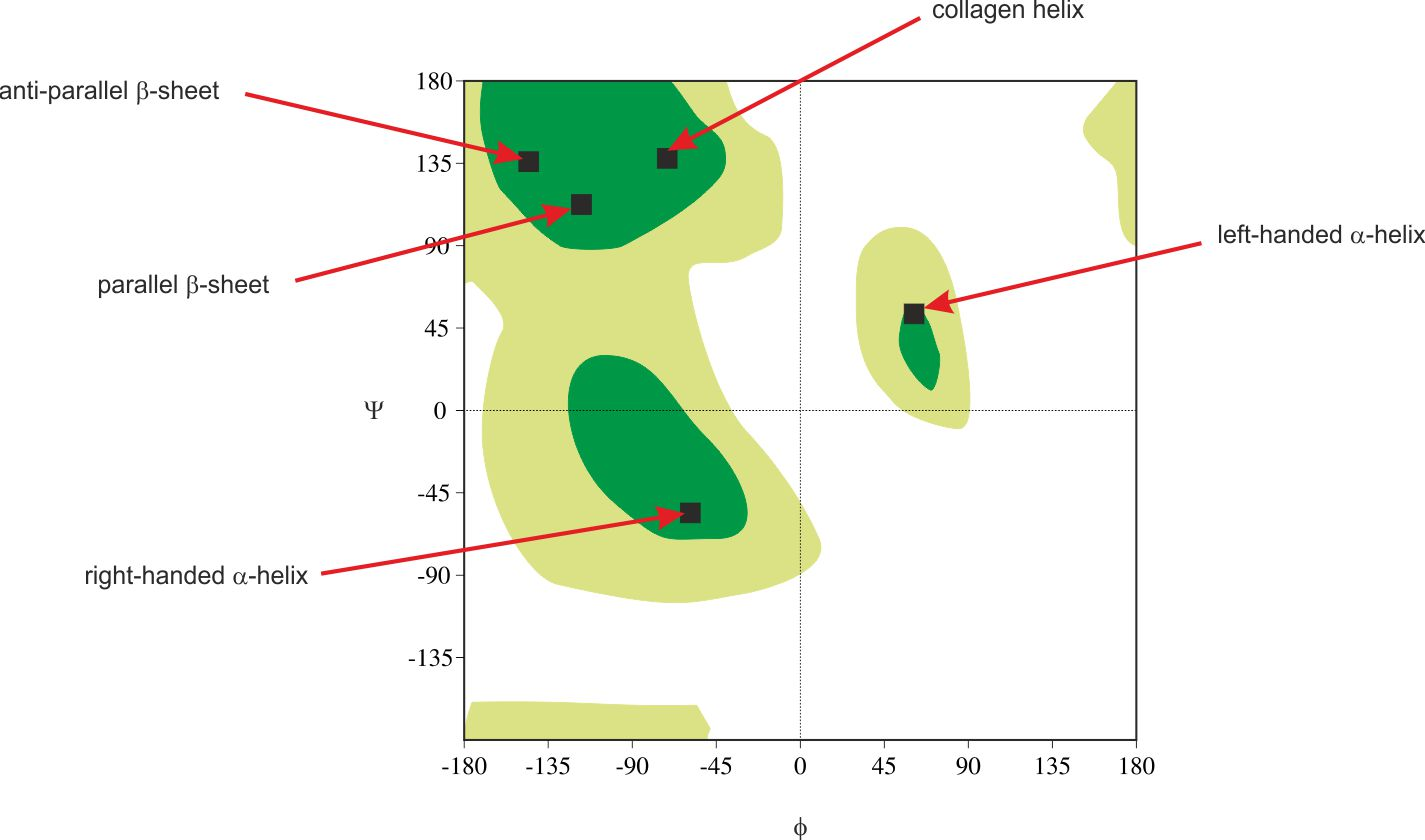
Where are anti-parallel beta sheets represented on the plot?
Upper left closer to y-axis
Where are parallel beta sheets represented on the plot?
Lower part of beta sheet area closer to y-axis
Where are right handed a-helices represented on the plot?
In portion below beta sheet area closer to y-axis
Where are left handed a-helices represented on the plot?
In portion that is slightly central further from y-axis (very rare)
What are the dihedral angles in an a-helix to form circular arrangement?
ψ = -60º φ = -60º to -120º
Helical structures make the peptide chain more compact. What are the number of residues per turn and the length?
3.6 residues per turn; 5.4 Å per turn
Why is the a-helix a favored structure?
It allows maximal hydrogen bond interaction between the peptide backbone without R-group steric clash
Polypeptide backbone forms the core of the helix with R chains protruding out
In what ways to b-sheets differ from a-helices?
-β-sheet is a more extended structure than the compact α-helix
-a minimum of two β-sheet strands are required for this structure
How are the strands in b-sheets connected and why are they oriented this way?
The two β-sheet strands form hydrogen bond contacts between the peptide N-H and peptide C=O
Orientation avoids R-group steric clash
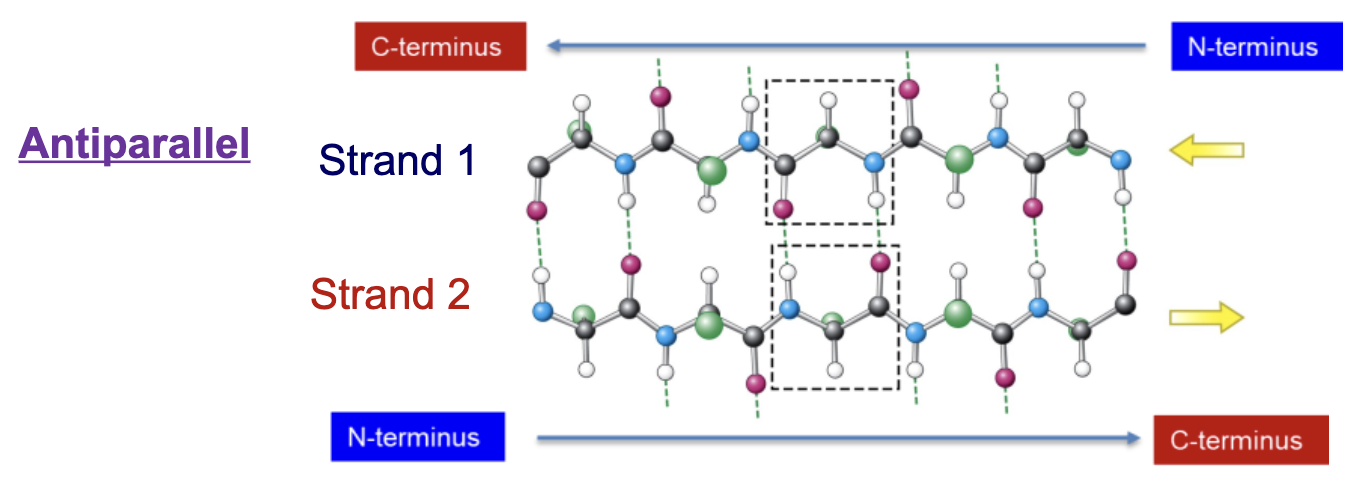
Describe the orientation of strands in an antiparallel b-sheet.
One strand runs from C-terminus to N-terminus while the other runs from N-terminus to C-terminus
Bonds are formed directly between peptide C=O and peptide N-H, creating a straight line
Describe the orientation of strands in a parallel b-sheet.
Both strands run in the same direction (both N-terminus to C-terminus or vice versa)
Bonds are formed between the peptide C=O and the peptide N-H, but these bonds are more diagonal than straight up and down.
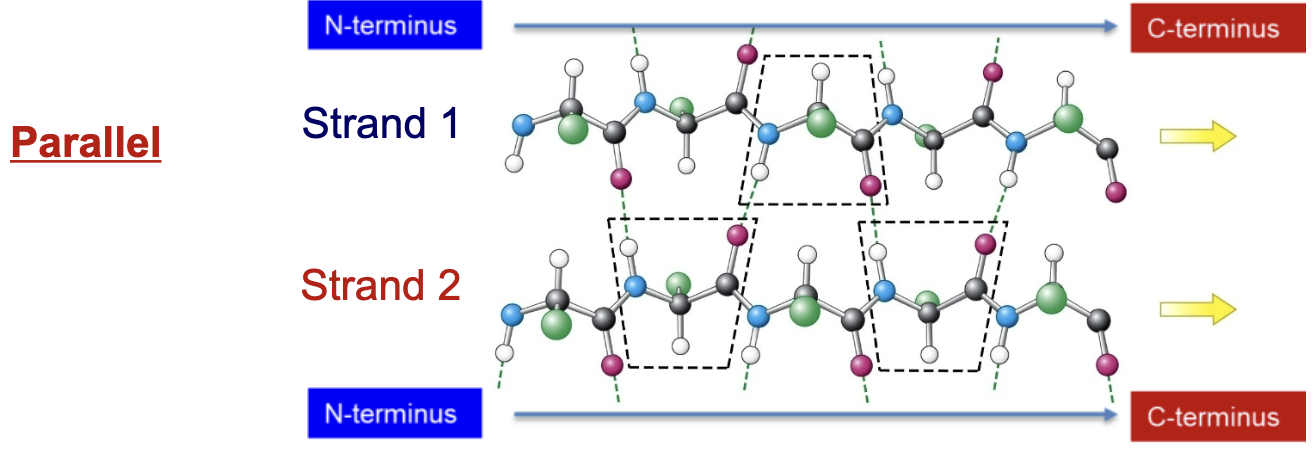
In ribbon diagrams, b-sheets are represented by flat arrows. What terminus does the arrow point towards?
The C-terminus
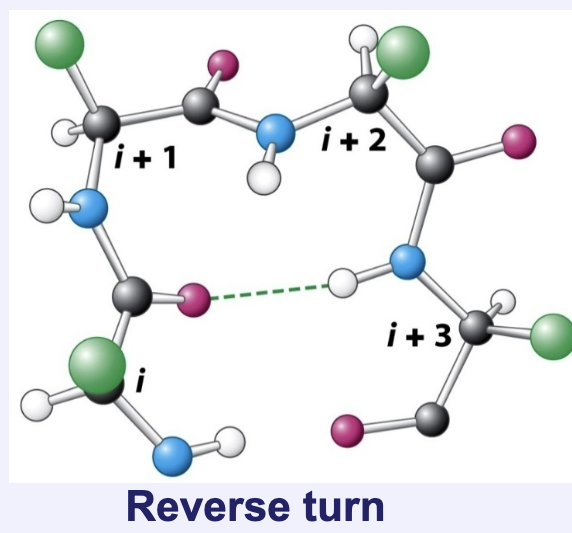
What are reverse turns/loops? What are they necessary for?
Special cases in which the polypeptide chain reverses direction
Occur between secondary structure elements and are essential for protein folding
Describe a reverse turn and what amino acids are found in high amounts in a reverse turn and why.
Four amino acid motif with high amounts of proline and glycine to accommodate extreme ψ and φ angles necessary for a turn
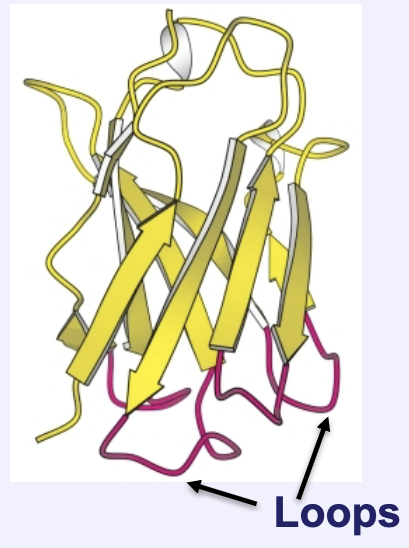
Loops are similar to reverse turns. How do they differ?
Loops perform the same function but often have more amino acids
The amino acid composition of the polypeptide determines the preference for α-helix, β-sheet, or reverse turn. What amino acids are most often found in a-helices?
Glutamate, Alanine, Leucine, Methionine, Glutamine, Lysine, Arginine, Histidine
REALM HQ, K?
The amino acid composition of the polypeptide determines the preference for α-helix, β-sheet, or reverse turn. What amino acids are most often found in b-sheets?
Valine, Isoleucine, Tyrosine, Cysteine, Tryptophan, Phenylalanine, Threonine
WTF You Can’t View It
Why do Y, W, F, and C prefer b-sheets?
large bulky side chains or contain a bulkier atom (sulfur) on β-carbon. b-sheets allow for less steric hindrance.
Why do I, V, and T prefer b-sheets?
β-branched carbon in R group, more steric clash in α-helix
The amino acid composition of the polypeptide determines the preference for α-helix, β-sheet, or reverse turn. What amino acids are most often found in reverse turns?
Glycine, Asparagine, Proline, Serine, Aspartate
No Damn GPS
Why does G prefer neither b-sheets or a-helices?
side chain H is small and flexible entropically not favored in either secondary structure. Extreme dihedral angles favors reverse turn
Why does P prefer neither b-sheets or a-helices?
side chain linked to alpha N, has no N-H to H-bond and has restricted Φ and Ψ angles
Why do D, N, and S prefer neither b-sheets or a-helices?
H-bonding side chains compete directly with backbone H-bonds
Consider a peptide sequence rich in leucine and alanine. Would you expect it to favor an α-helix or β-sheet? Why?
It would likely favor an a-helix because the side chains of L and A are relatively small, so the steric hindrance caused by a compact a-helix structure does not affect formation. Also, their side chains are nonpolar/hydrophobic, meaning they do not interfere with the C=O to N-H hydrogen bonds in the interior of the helix that stabilize its structure.
A student proposes that glycine-rich sequences would form stable α-helices because glycine is small. Do you agree or disagree? Explain using backbone flexibility and entropy.
Disagree because the flexibility of glycine (can have extreme dihedral angles) due to its small R-group would cause flexibility in the backbone, leading to overall instability.
The formation of a-helices causes a loss in entropy. This is okay for other AA’s, but glycine can have many possible conformations, making the loss of entropy much greater and unfavorable.
Why does proline often disrupt an α-helix, and in what structural context could proline be useful instead?
Proline’s amine group cannot form H-bonds due to being in a ring, which would destabilize the helix. Also, proline’s angles are very restricted as a result of its ring structure, making helical formation difficult because the necessary dihedral angles are not possible. Proline would likely be more useful in reverse turns and loops.
Predict whether a peptide rich in valine and isoleucine is more likely to form α-helices or β-sheets. Justify your answer with R-group sterics.
The peptide would likely form b-sheets because the R-groups of V and I are bulky, which would cause steric hindrance. This steric hindrance would make a-helix formation energetically unfavorable. b-sheets provide more room for larger R-groups, reducing steric hindrance.
In an antiparallel β-sheet, hydrogen bonds are more linear than in parallel β-sheets. How might this difference affect the relative stability of the two structures?
This would likely cause antiparallel b-sheets to be more stable, as strong H-bonds tend to be linear. This indicates the H-bonds in an antiparallel b-sheet are stronger, making it more stable.
If most Φ and Ψ angles are forbidden due to steric clashes, why are glycine and proline often exceptions? How does this affect where they are found in a Ramachandran plot?
Glycine has a very small R group (a single H), which allows it greater flexibility as well as minimal steric clash. Because of this, glycine is often found in the “forbidden” area of the plot. Proline has a very constrained ring structure, which restricts angles. This makes proline’s range on the plot very narrow.
Looking at a Ramachandran plot of a protein, you see clusters of residues in both the α-helix and β-sheet regions. What does this suggest about the protein’s secondary structure composition?
It likely has a mixed composition, containing both b-sheets and a-helices
Why are proline and glycine commonly found in β-turns? Give an example of how loops or turns are essential in connecting secondary structure elements in a functional protein
Proline has restricted angles, causing it to often be found in “kinks” or turns in protein structure. Glycine has greater flexibility due to its small R-group, allowing it to take on angles that are typically forbidden. This allows it to be found in loops/turns. Because loops and turns connect secondary structures, they are necessary for compact protein folding.
Calmodulin contains both α-helices and β-sheets. Why might a protein with multiple secondary structure types be more versatile in binding diverse partners than one with only α-helices?
Because diversity in structure allows for a wider range of interactions with different molecules.
α-helices present cylindrical, often amphipathic surfaces that can wrap around or insert into partner proteins.
β-sheets provide flatter, extended surfaces that can form large interaction interfaces