Chemistry MCAT - Johan
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
acidity
increases from upper left to lower right (different!)
1. down a group, size explains: larger atom means conjugate base (cation) is more stable
2. across a period, electronegativity explains: more protons means conjugate base (cation) is more stable
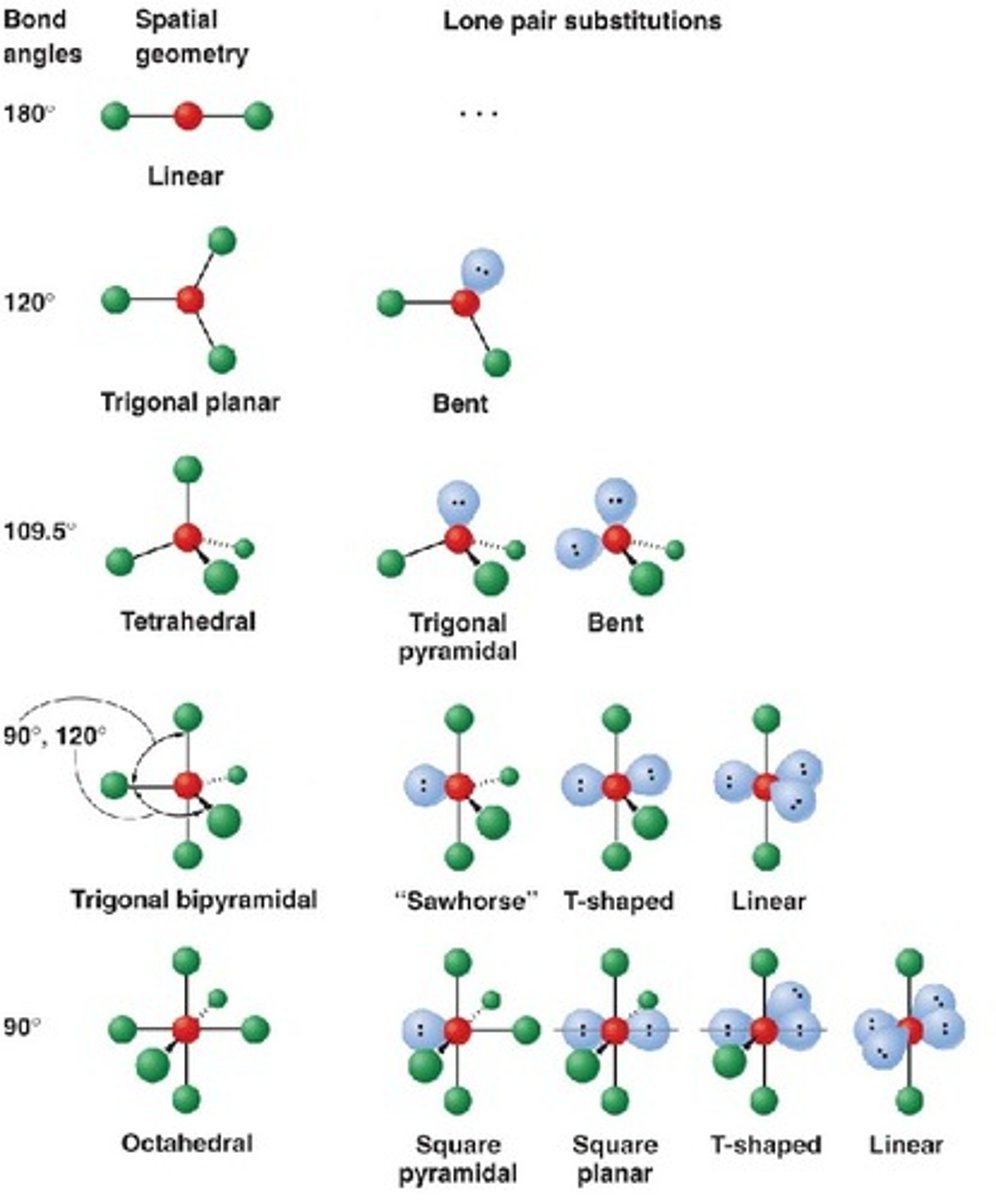
hybridization
sp for 2 groups, sp2 for 3 groups, sp3 for 4 groups
lone pairs determine molecular geometry: NH3 is trigonal pyramidal (sp3), H2O is bent (sp3), XeF4 is square planar (sp3d2), SF6 is octahedral (sp3d2)
octahedral is 6 bonds, 90 degrees (sp3d2)
sp3 to sp2 conversion to attain planarity and aromaticity (4n+2 electrons resonating in a ring)
amide N can convert from sp3 to sp2 to get resonance stabilized by carbonyl O
orbitals with more s character are more stable
bond length
depends on atomic radius (which increases to lower left)
depends on bond order, more electrons shared is a stronger bond and closer bond
ATP phosphates
alpha- phosphate closest to sugar
beta- middle one
gamma- phosphate on tip
covalent bonds
metal and nonmetal share electrons
electronegativity differences creates dipoles at each bond
molecular dipole found by adding up all the bond dipoles
metallic bonds
sea of electrons delocalized between metal ions
usually S and D block elements
these compounds are conductors and malleable
coordinate covalent bond
formed between atoms with lone pairs and atoms that are electron deficient
usually between transition metals and organic compounds, where compounds donate electron pairs to coordinate with the metal
coordinate number- number of compounds coordinating with metal ions
Fe2+ can form 6 coordinate covalent bonds (that completely fill up its valence shells)
Fe2+ forms coordinate covalent bonds with hemoglobin
ionic bonds
formed between cations and anions
ions dissociate in aqueous solution, become conductor
as solids, these compounds are insulators and brittle
insulator/conductor
insulator- valence electrons tightly bound to atom
conductor- delocalized electrons, metallic bonds
intermolecular forces
pulling apart atoms is always endothermic!
4 types of IMFs:
1. ion-dipole forces- ions and polar molecule
2. dipole-dipole forces- two polar molecules, align along the molecular dipoles, H-bonding is special case
3. dipole-induced dipole forces- polar, nonpolar molecules
4. london dispersion forces- Van der Waals, temporary
5. Hydrogen bonds- align along the bond dipoles, require a donor and an acceptor, only N, O, and F can do hydrogen bonds
solvation shell
A cagelike network of solvent molecules that forms around a solute in a solution
decrease in entropy
active site
acidic and basic amino acids can undergo H-bonding and ionic interactions with the substrate
ATP has negatively charged phosphates that interact well with H, R, and K
entropy
disorder, always increasing in universe
what increases entropy (S):
1. increasing number of particles
2. increasing volume
3. increasing temperature
formation of a more organized compound or state would decrease entropy
enthalpy
breaking bonds is endothermic (dH > 0)
forming bonds is exothermic (dH < 0)
heat of formation (dHf) of elements in standard state is 0
heat of reaction (dH) = Hf products - Hf reactants
multiply by number of moles
positive dH means endothermic, heat is reactant
negative dH means exothermic, heat is product
Gibbs free energy and spontaneous reactions
dG = dH - TdS
dGo = - RTlnK
dG = dGo + RTlnQ
free energy of formation (dGf) of standard state elements is 0
spontaneous process is exergonic (dG < 0)
nonspontaneous process is endergonic (dG > 0)
examples:
combustion (-dH, +dS) is spontaneous at all temperatures
freezing (-dH, -dS) is spontaneous at low temperatures
ATP -> ADP (+dH, +dS) is spontaneous at high temperatures
bond and IMF strengths
order of bond strengths:
1. covalent
2. ionic
3. metalic
4. coordinate covalent
order of IMF strengths:
1. ion-dipole
2. dipole-dipole (H-bonds)
3. dipole-induced dipole
4. london dispersion forces
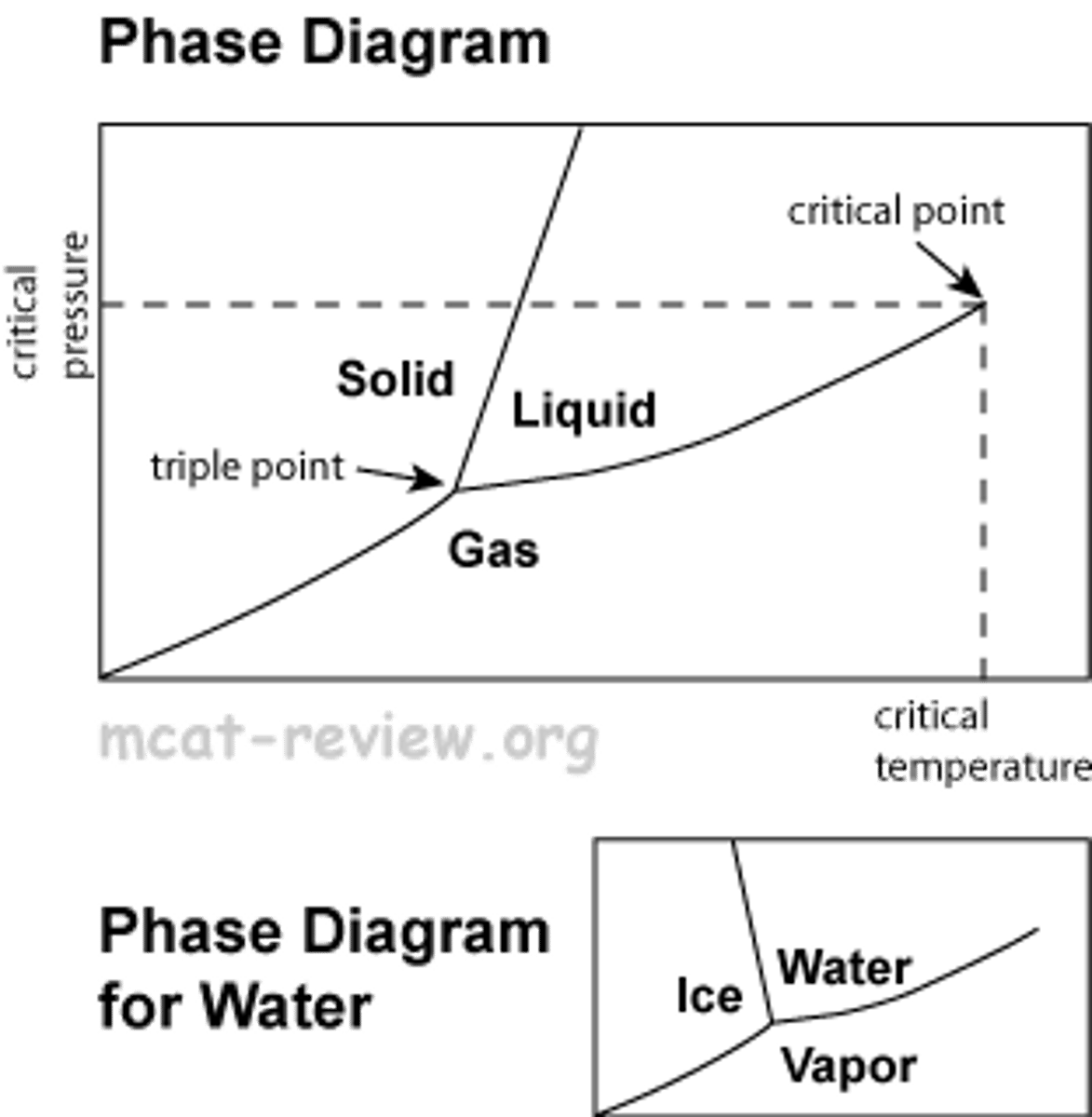
phase diagram
as pressure and temperature increase, phase change from solid to liquid to gas
triple point- all three phases coexist
critical point- above point liquid and gas are no longer distinct, becomes supercritical fluid
sublimation and evaporation line up on the diagonal
melting line is positive sloping, but for water it is negative sloping (MP decreases when pressure increases, since ice has more volume than water)
density is directly proportional to external P, indirectly proportional to external T
kinetic-molecular theory of gases
an ideal gas has:
1. no IMFs
2. particles have no volume
3. temperature is average KE
4. elastic collisions with container
high temperature, low pressure creates an ideal gas since interactions are minimized
units of pressure
1 atm = 100 kPa = 760 torr = 760 mmHg
Graham's law of diffusion/effusion
rate1/rate2 = sqrt(molar mass2/molar mass1)
heavier particles diffuse slower
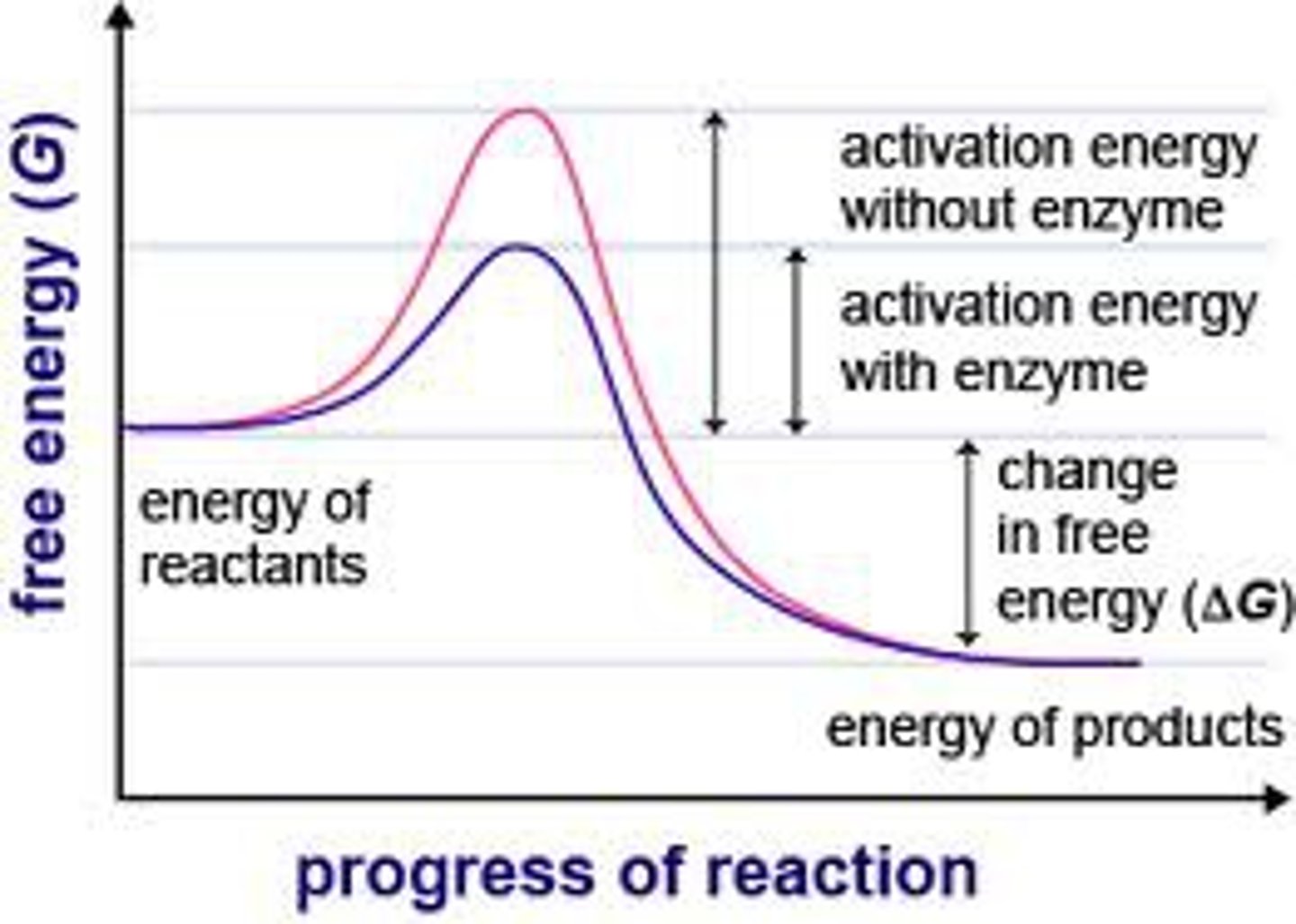
reaction coordinate graph
x-axis is reaction progression
y-axis is free energy
peaks are transition states
valleys are intermediates
the highest peak is the rate limiting step
difference between reactant energy and highest peak is Ea
catalysts lower peaks, does not affect equilibrium

reaction rate
factors that increase it:
1. higher concentration of reactants
2. proper orientation, catalysts help
3. minimum energy to overcome Ea
rate constant
factors that increase it:
1. increased temperature
2. decreased activation energy
3. not affected by concentration of reactants!
rate law
rate = k[A]^x[B]^y
order is sum of exponents
determine rate law:
1. coefficients of reaction become the exponents of rate law
2. solids are not included
3. rate law determined by rate limiting step
determine rate law from data:
1. determine rate constant from any trial
2. 0th order if rate is same between two trials with diff. concentrations of a reactant
3. 1st order is rate increase is same as concentration increase of reactant
4. everything else must be 2nd order
catalytic efficiency
vmax = k_cat*[total enzyme]
catalytic efficiency = k_cat/Km
equilibrium constants
K is conc. of reactants over products, K > 1 favors products
K only changes when temperature changes
Keq > 1 means dG < 0, so spontaneous
Keq < 1 means dG > 0, so non-spontaneous
Q is reaction quotient using present concentrations state:
1. Q > K favors shift to reactants
2. Q = K is in equilibrium
3. Q < K favors shift products
why? because of the equation below:
1. dG = dGo + RTlnQ
2. dGo = -RTlnK
3. dG = 0 at equilibrium
factors that affect equilibrium
Le Chatelier's principle says system in equilibrium shifts to minimize stress:
1. change in concentration/partial pressure
2. change in pressure/volume
3. change in temperature
change in concentration or partial pressure- causes shift to reduce the change, solids do not affect equilibrium!
increase in pressure- causes shift towards side with less moles of gas
decrease in volume- causes shift towards side with less moles of gas
change in temperature- treat heat as a reactant/product
solubility equilibrium
Ksp- concentrations of products since the reactant is solid
molar solubility- moles of solid dissolved, you can plug this into Ksp equation
Qsp > Ksp means that oversaturated, precipitate forms (shift towards reactants)
common ion effect- salt solubility decreases when mixed in solution with a common ion (shift towards reactants)
opposite effect is true, salt solubility increases when mixed with solution that reacts with an ion, like an acid/base (shift towards products)
association/dissociation equilibrium
Ka = [AB]/[A][B]
Kd = [A][B]/[AB]
kinetics vs. thermodynamics
kinetics- rate, intermediate, activation energy, catalyst
faster product
thermodynamics- stability, equilibrium, spontaneity, entropy, enthalpy, free energy
more stable product
for example, free energy of ion transport does not tell you about the kinetics of a channel protein
acid/base trends
acids have more electronegative atoms
atoms without H can be acids if electron deficient
increased acidity:
1. more positive charge
2. more electronegative atom (only within a period)
3. larger atom (only within a group)
bases have less electronegative atoms
atoms without lone pairs are not basic
increased basicity:
1. more negative charge
2. less electronegative atom
3. smaller atom
acidity/basicity of salts
salts will completely dissociate in water!
acidic salt contains ion that is a weak acid
cations in group I and II are not acidic (spectators!)
basic salt contains weak base
anions like Cl, Br, or I are not basic (spectators!)
determine pH of solution
1. The acidity of an element increases as one moves to the right or down the periodic table.
2. The conjugate base of a strong acid is a very weak base, forming pH neutral solutions.
3. The conjugate base of a weak acid is a kinda weak base, pH will be higher.
amphoteric
can be both acid or base
examples:
1. amino acids
2. water
3. bicarbonate
strong acids (6) and calculating pH
HCl, HBr, HI, H2SO4, HClO4 (perchloric acid), HNO3
how to calculate pH:
1. write the concentration in scientific notation
2. take the negative of the 10 exponent
3. round down (because you ignored the actual value) and say it's somewhere in between those two values
for diprotic acids, just double the concentration, you can get a negative pH
large Ka, weak conjugate base has small Kb
weak acids and calculating pH
acetic acid, H3PO4 (phosphoric acid), H2CO3 (carbonic acid), NH4, HF
Ka = [H+]^2/[HA] from ICE table
pH = -1/2log(Ka[HA])
use same estimation technique
small Ka, strong conjugate base has large Kb
pH in solution is a bit under 7
weak acids can fully dissociate just like strong acids if another reaction removes protons to shift equilibrium to the right (like bicarbonate buffer)
buffers
conjugate acid/base pair, minimize pH change
weak acid or base (acetic acid, phosphoric acid, carbonic acid are weak acids, NH3 is weak base etc.)
both acid and base must be available to push equilibrium to account for changes in conc.
pH = pKa + log([A-]/[HA])
HH equation shows that conc. of acid/base dependent on pH
if pH < pKa, then buffer is more protonated, each difference of 1 in pH is 10-fold increase in concentration
adding water dilutes your buffer, reduces buffering capacity, but does not affect equilibrium
choose a buffer within 1 of the pH you want to maintain!
![<p>conjugate acid/base pair, minimize pH change</p><p>weak acid or base (acetic acid, phosphoric acid, carbonic acid are weak acids, NH3 is weak base etc.)</p><p>both acid and base must be available to push equilibrium to account for changes in conc.</p><p>pH = pKa + log([A-]/[HA])</p><p>HH equation shows that conc. of acid/base dependent on pH</p><p>if pH < pKa, then buffer is more protonated, each difference of 1 in pH is 10-fold increase in concentration</p><p>adding water dilutes your buffer, reduces buffering capacity, but does not affect equilibrium</p><p>choose a buffer within 1 of the pH you want to maintain!</p>](https://knowt-user-attachments.s3.amazonaws.com/690aea88-6dc7-4a80-82ad-c851f61e06c8.jpg)
henderson-hasselbalch equation
pH = pKa + log [base]/[acid]
conc. of acid/base are dependent on pH
calculate pH of buffer solution given conc.
when [base] = [acid], pH = pKa at half-equivalence point
auto-ionization of water, pH/pOH
at standard conditions:
Kw = KaKb = 1e-14
pKa = -logKa, pKb = -logKb
pKa + pKb = 14 (pH + pOH = 14)
pH = 0 is most acidic, pOH = 0 is most basic, neutral is pH = 7
titration
sigmoidal curve, indicator changes color at equivalence point
used to determine concentration of solution, where moles added equals moles in solution to reach each equivalence point
at equivalence point:
1. compound becomes deprotonated
2. moles of acid = moles of base, meaning everything is neutralized
3. center of vertical segments
at half-equivalence point:
1. pKa = pH
2. [acid] = [base], moles of acid = 1/2 moles of base, meaning half of the acid is neutralized
4. center of horizontal segments
polyprotic titration can be done with amino acids, double the moles added to reach the second equivalence point
for titrating a strong acid, the same volume of strong or weak bases is required to neutralize, the strength will only affect the pH of equivalence point
![<p>sigmoidal curve, indicator changes color at equivalence point</p><p>used to determine concentration of solution, where moles added equals moles in solution to reach each equivalence point</p><p>at equivalence point:</p><p>1. compound becomes deprotonated</p><p>2. moles of acid = moles of base, meaning everything is neutralized</p><p>3. center of vertical segments</p><p>at half-equivalence point:</p><p>1. pKa = pH</p><p>2. [acid] = [base], moles of acid = 1/2 moles of base, meaning half of the acid is neutralized</p><p>4. center of horizontal segments</p><p>polyprotic titration can be done with amino acids, double the moles added to reach the second equivalence point</p><p>for titrating a strong acid, the same volume of strong or weak bases is required to neutralize, the strength will only affect the pH of equivalence point</p>](https://knowt-user-attachments.s3.amazonaws.com/6fcfe5c1-4f53-4bcb-ae8a-3f03d47f6123.jpg)