Archaeological Ceramics Vocab [MIDTERM]
1/111
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
112 Terms
adsorption
The adhesion of atoms, ions or molecules to a surface. Water of plasticity is adsorbed water.
Synonyms: sticking to
absorption
The process by which one thing absorbs or is absorbed by another.
Synonyms: soaking up, drawing in
anisotropic shrinkage
different rates of shrinkage along two directions of measurement
varying alignments of particles may occur in parts of a vessel where the orientation of the clay platelets — and wall thickness — changes sharply, such as at corners, seams, and angles
aplastic
particulate matter in a clay body that does not contribute to plasticity or that reduces the plasticity of the clay
one of several terms used for temper, but lacking implications of either natural occurrence or deliberate addition by the potter
see also: additive, grog, inclusion, nonplastic, temper.
atmosphere (firing)
the gaseous composition of the air in the environment of heating and cooling clay articles, particularly with reference to the availability of oxygen
see also oxidation, reduction.
ball clay
a fine-textured, highly plastic secondary clay, usually composed of aged kaolinite mixed with other clay minerals
typically firing white or cream
used to make sturdy “white ware”
band
a design element or fundamental part that is continued or repeated along a straight line that, on pottery, most commonly encircles the vessel, but may also be vertical or diagonal.

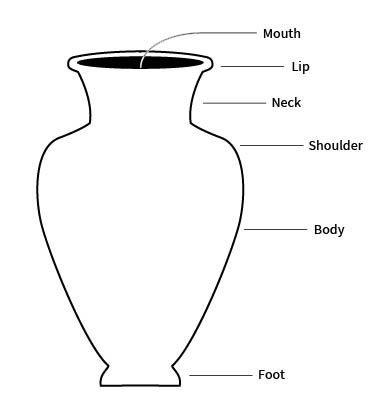
base
the underside of a vessel, or that part of a vessel in contact with the surface it rests on during normal use
sometimes called the foot
biscuit (bisque)
unglazed fired pottery, awaiting glazing
the first or preliminary firing of a ware that is subsequently glazed and refired in the glost firing
body (clay body)
clay or a mixture of clay and inclusions (temper) that is suitable for forming vessels or that has been fired into a vessel
often used interchangeably with fabric, ware, or paste; being closest to paste
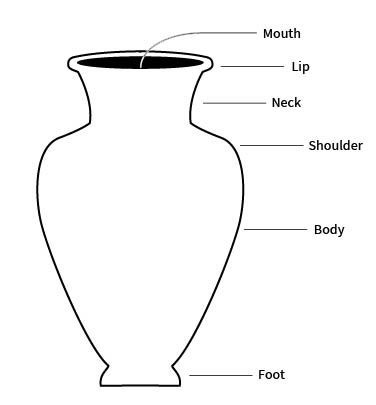
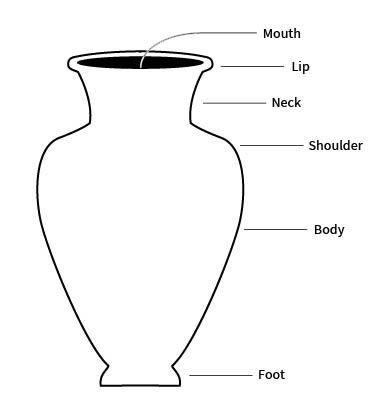
body
the portion of a vessel between the orifice and the base
also sometimes called the belly
burnish
a method of producing a luster on an unfired clay surface by rubbing it while leather-hard with a hard, smooth object to compact and align the surface particles

calcium carbonate
Chemical formula: CaCO3
It is a common substance found in rocks (limestone, marble) as the minerals calcite and aragonite, and is the main component of the shells of marine organisms, snails, and eggs.
casting
a process for forming a ceramic object by pouring a clay slip into a hollow, porous (usually plaster) mold and leaving it there long enough for a layer of clay to settle and thicken on the mold wall. The remaining slip is poured off, and the object is removed from the mold when it has dried.
Also called slip casting or solid casting.

carination
sharp change in direction (in wall structure)

cavetto
concave zone encircling a vessel (usually between carinations)

castellation
‘points’ around the rim that make a squared, rather than round vessel opening

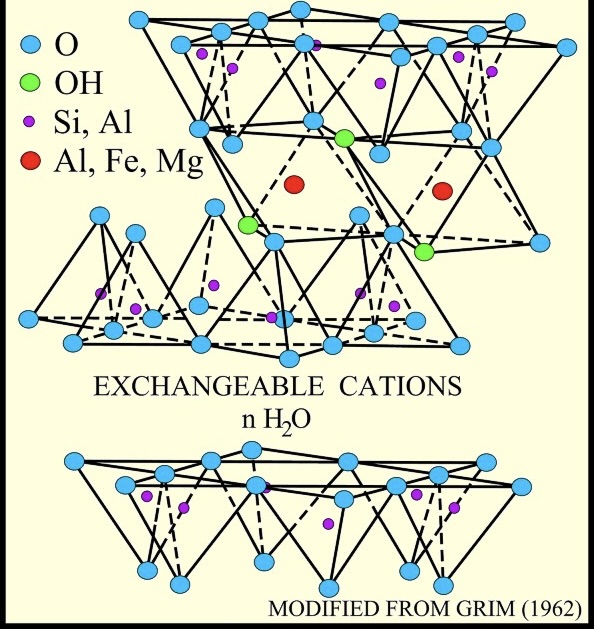
cation exchange capacity (CEC)
the ability of a material, such as a clay particle, to adsorb cations (positively charged ions) from the surrounding solution.
relates to the negative surface charge of the silicate platelets, and increases with organic content and as particle size is reduced.
ceramic(s)
objects manufactured of silicates (primarily clay) and formed by application of heat
in art and archaeology: high-fired, usually vitrified cooking and serving utensils and art objects
china clay
another name for kaolin clay
usually specifically a white-firing primary clay
clay
one of several hydrous alumina-silicate minerals formed by decomposition of rock and that have the property of plasticity
an extremely fine particle size grade, less than 0.002 mm in diameter
sedimentary materials composed of 35% - 40% particles in the extremely fine particle size grade
a fine-grained, naturally occurring material that becomes plastic and malleable when wet and hardens with the application of heat
clay body
all the stuff in the clay “recipe” includes clay & additives
refers to the actual clay mixture used for forming objects
often a mix of various kinds of clay and tempering agents, fluxes, etc...
coil fracture
a smooth-edged circumferential breakage characteristic of coiled vessels in which the coils were poorly bonded, resulting in planes of weakness

coiling
a method of hand building a clay pot by successive additions of ropes or coils of clay
variants include ring building, spiral coiling, and segmental coiling
collar
a raised and extended orifice that begins at or just above the point of maximum diameter of the vessel and does not significantly reduce the opening relative to the body diameter
colorant
a chemical element that contributes color to a mixture
see also: pigment, stain
cones (pyrometric cones)
small, elongated pyramids made of controlled mixtures of ceramic materials in a numbered sequence that soften and bend when heated under specific conditions
when cones are placed in a kiln during firing, their bending provides an index of heat treatment and proper firing

configuration
the arrangement of decorative motifs on a vessel to fill a spatial division and form the design
core (coring)
a black or gray zone in the interior cross section of a vessel wall, usually associated with incomplete removal of carbonaceous matter from the clay during relatively low-temperature firing
not to be confused with black coring at high temperatures, which results from trapped gases and may lead to bloating

crystal lattice water
chemically combined water (as hydroxyls) held as an integral part of tiered crystalline structure of day minerals, normally lost only with heating Ae clay temperatures between 450°C and 600°C
distinct from interlayer water
crystalline structure
the regular arrangement of the atoms of a mineral
diagenesis
The sum of all processes that cause change in a sediment, after its deposition, but before its final lithification
e.g. the chemical alteration of a feldspar to form a distinctly new mineral: i.e. a clay mineral
dehydroxylation
removal of OH- ions (chemically combined water) from the crystalline structure of minerals, as in the dehydroxylation of clays by application of heat
docking
soaking a freshly fired article in cold water for a short period to prevent or reduce spalling of the ware if it contains calcareous inclusions
dunting
cracking that occurs in a fired ware as a result of thermal stresses
More specifically, cracking that occurs if a ware is cooled too rapidly or that appears on refiring bisque ware through 400 - 600°C, with the expansion of quartz.

earthenware
a glazed or unglazed non-vitreous ceramic material, usually low-fired, porous, and permeable, and red or brown in color.
Categories include:
coarse earthenware (e.g., construction materials, bricks)
fine earthenwares (e.g. majolica)
elastic deformation
temporary, reversible deformation of a material from an external force
extensibility
the amount of deformation a clay can withstand (in millimeters) beyond the yield point before cracks appear
fabric
the composition of a fired ceramic, including clay, inclusions, and pores and excluding surface treatment
often used synonymously with body, paste, or ware
film water
water that surrounds and separates platelets in a clay/water mass and makes the mass plastic
see also: shrinkage water
firebox
the combustion chamber of a kiln (usually one fired by wood), typically beneath the ware chamber
fire clay
a type of refractory clay found deep in the earth often under coal deposits (a residual kaolinite/primary clay that is high in alumina >30%) used for refractory materials like furnace linings, bricks, crucibles, kilns, etc…
can withstand very high temperatures; melting point above 1,600 C° (~3000 F°)
firecloud
a darkened area on a vessel's surface resulting from uneven firing and the deposit of carbon in the pores during firing
characteristic of firings in which fuel and vessels are in immediate proximity

flux
a substance in a clay body, slip, or glaze that lowers the melting point of the mixture and promotes vitrification
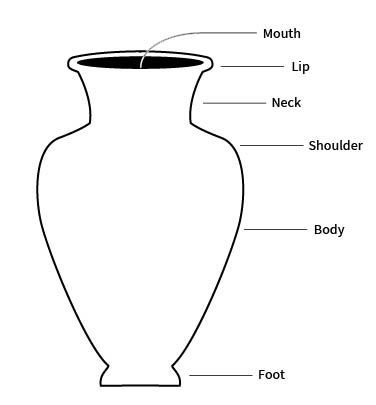
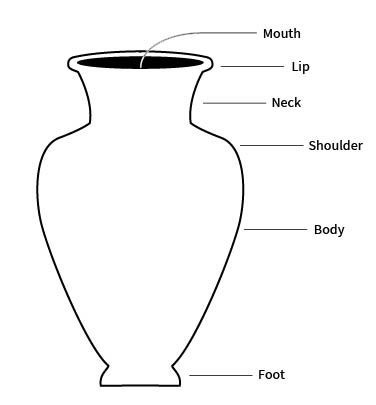
foot
the base of a ceramic vessel
usually a ring-like projection formed by tooling or by adding a coil
glaze
a glassy coating melted onto the surface of a ceramic article
applied as a liquid suspension to a ware that has usually been fired once (biscuit) and is subsequently refired
green
formed but unfired ceramic articles or the properties
used as in greenware, green strength, etc
green strength
the ability of a formed and dried piece to withstand mechanical stress without deformation
grog
pre-fired clay (or old pottery) that is crushed or ground to a small particle size and then added to a clay as a type of temper to modify its properties
hygroscopic
the ability or tendency of a material (e.g., CaO) to take up moisture readily from the surrounding air or other moist materials
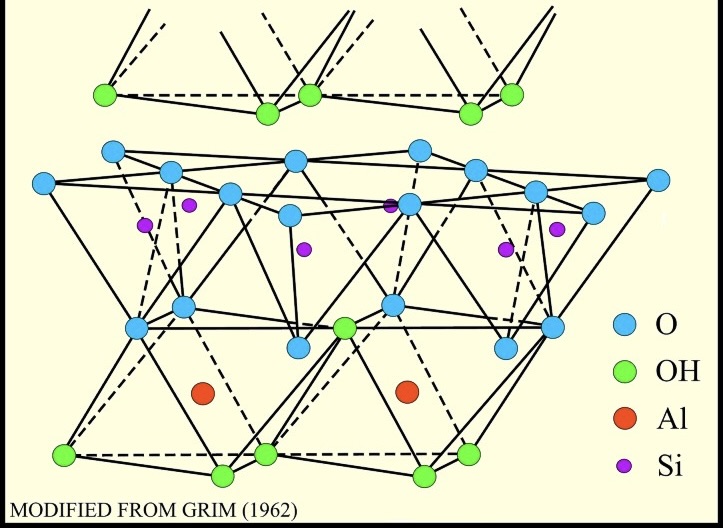
illite
a group of clay minerals having a three-layer, non-expanding structure similar to that of well-crystallized micas
Chemical Composition: (K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2 • (H2O)]
(I know it's long, this is the simplest one I could find)
![<p>a group of clay minerals having a <strong><u>three-layer</u></strong>, <strong><u>non-expanding</u></strong> structure similar to that of well-crystallized micas</p><ul><li><p>Chemical Composition: <strong>(K,H<sub>3</sub>O)(Al,Mg,Fe)<sub>2</sub>(Si,Al)<sub>4</sub>O<sub>10</sub>[(OH)<sub>2 </sub>• (H<sub>2</sub>O)]</strong></p><ul><li><p><em>(I know it's long, this is the simplest one I could find)</em></p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/80acba98-63f0-4ece-bf9d-05f1867ba3ac.jpg)
inclusion
particulate matter, usually mineral in nature, present in a clay or fabric either naturally or added by the potter
often used synonymously with temper
interlayer water
water that exists between the unit layers of the three-layer (or 2:1) clay minerals
the last water to be lost in drying
inversion
a transformation or change of phase, typically in a solid, from one polymorphic form to another with different atomic structure and bonding
see also: quartz inversion
ion
an atom or compound with an electrical charge
either positive (cation) or negative (anion)
isomorphous substitution
when cations (like Si or Al) in the octahedral or tetrahedral sheet are replaced by different kinds of cations (like Mg), without change in crystal structure
kaolinite
a common clay mineral having a two-layer structure of silica and alumina
Chemical Composition: A12O3 • 2SiO2 • 2H2O
the principal mineral of the kaolin clay (also called china clay) group.
kiln
an enclosed or partially enclosed chamber(s) for firing ceramic materials
may be classified on the basis of characteristics of construction, firing characteristics, or products
leather-hard
clay that is dried to the critical point so that individual clay particles touch and the body is rigid, but also retains sufficient moisture to be carved or joined
levigation
separating fine from coarser material, such as clays, by mixing with a liquid (water) and washing down a series of traps, allowing the coarser material to settle
lime popping (spalling)
a surface defect on ware containing inclusions of calcium carbonate (CaCO3), as limestone, shell, or calcite)
When fired to temperatures between about 650°C and 900°C, the inclusions decompose and after cooling the lime (CaO) rehydrates with an accompanying expansion

lip
the edge or margin of the orifice of a vessel
sometimes refers more specifically to a modification of a rim of a vessel for pouring
maturity
the range of temperature and time (the maturing range) at which a clay body fires to desired qualities of hardness, porosity, and serviceability
the temperature and time at which a glaze develops qualities of bonding (to the body), stability, strength, and texture
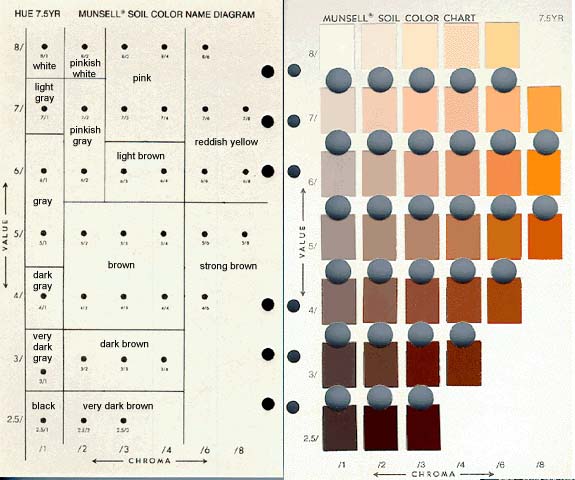
Munsell chart
a series of charts published by the Munsell Color Company for the standardized identification and description of colors
color is divided into three components, hue, value, and chroma, and individual shades, tints, and tones are represented by rows of small color chips
neck
the part of a vessel between the shoulder and the rim, typically characterized by a marked constriction of the maximum body diameter
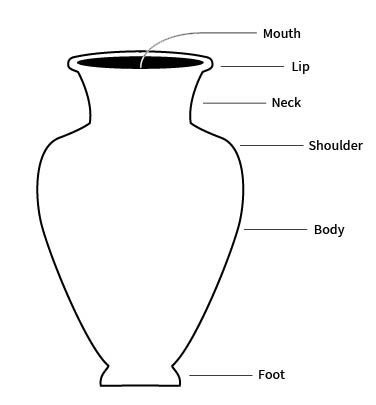
orifice
the mouth or opening of a vessel, usually hollowware
oxidation
a firing atmosphere characterized by an abundance of free oxygen, which combines with elements (such as iron) in the body and yields clear colors of the ceramic body
see also: reduction
oxide
a compound of oxygen plus another element
parting agent
a material, such as sand, ash, or dry clay, sprinkled over a mold or working surface to prevent wet clay from sticking
paste
a clay or mixture of clay and added materials, often used synonymously with fabric, body, or ware
Technically this differs from fabric because it does not include pores and differs from ware because it excludes surface treatment
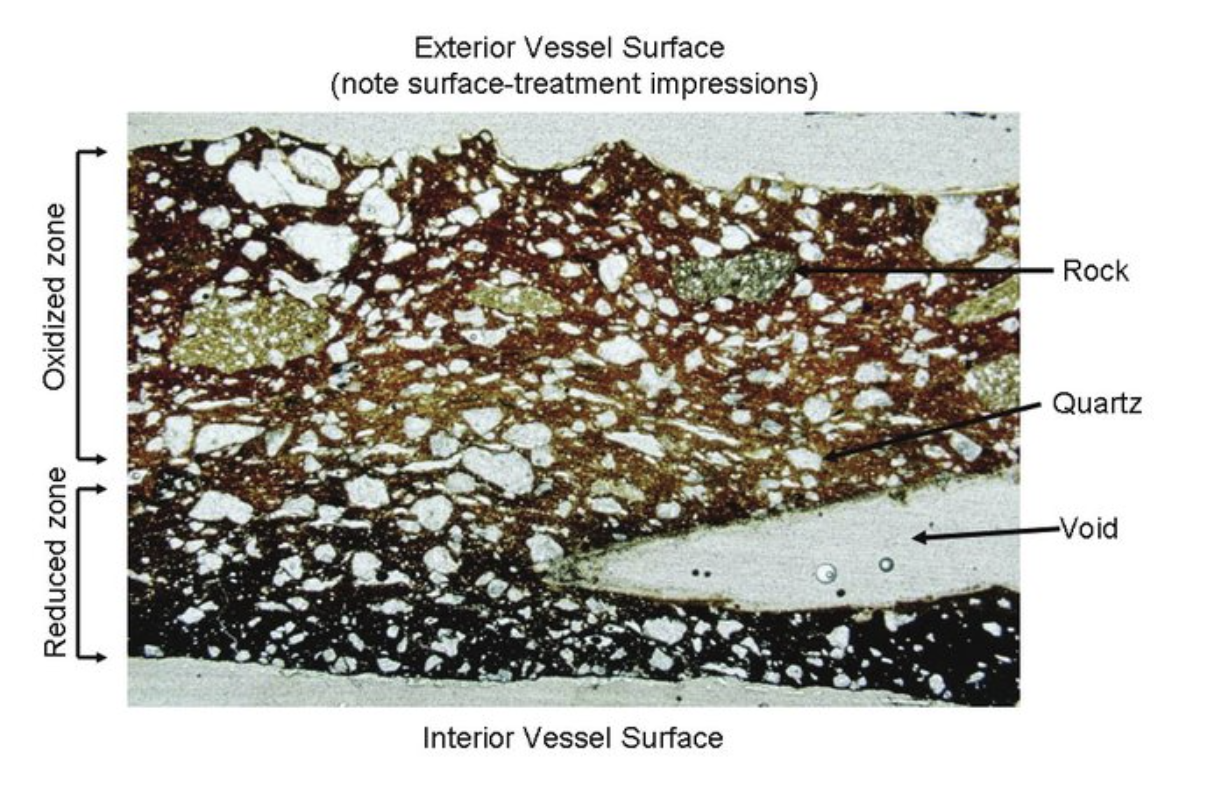
petrography
the microscopic study and description of rocks or other mineral based materials (such as ceramics) by their optical properties
involves thin sections and polarized light microscopy
phyllosilicate
a layered silicate mineral
the major category of clay minerals, composed of those with a regular ordering of layers of silica and alumina structural components
the prefix ‘phyllo’ in Greek means ‘leafy’ or ‘leaf-like’, which, in this context, refers to the tetrahedral and octahedral sheets in their crystal structure
plastic deformation
stress exceeding the elastic limit, causing permanent shape distortion
plasticity
the property of a material, such as a clay, that enables it to be shaped when wet and to hold this shape when the shaping force is removed
combines the strength of a solid with the fluidity of a liquid
plasticity, water of (water of plasticity)
the water required for a clay material to develop optimal plasticity
pica
a craving to eat something that is not regarded as nutritive, such as ice, starch, clay, or chalk
porcelain
a fine, vitrified, high-fired ceramic body, usually translucent and white, used for pottery or various industrial and specific products
pore water
mechanically combined water remaining in the pores and capillaries of a clay article after shrinkage is completed and particles come into contact with each other
primary clay
a clay deposit located on its geological site of formation by weathering from a parent rock (as opposed to a secondary or transported clay)
see also: residual clay
quartz inversion
a change in the atomic structure and bonding of quartz during heating
One inversion (from a to P) occurs at 573°C, the second (to tridymite) at 867°C, and the third (to cristobalite) at 1250°C
reducing atmosphere
an atmosphere of firing in which oxygen is removed from the clay or glaze
lacks free oxygen and is frequently smoky because of reducing agents such as carbon, or carbon monoxide
reduction
result of incomplete oxidation due to a reducing atmosphere
a dark, often vitrified, vessel wall interior is a good sign of carbon trapping; i.e. reduction
refractory
ceramic materials, usually high in alumina and silica, that can withstand extremely high temperatures and are slow to melt
often called “fire clays”
melting point above 1,600 C°
residual clay
a clay deposit near the parent rock from which it weathered; aka primary clay (as distinguished from a secondary clay)
see also: primary clay
rilling
the spiral ridges or striations around the interior or exterior surface of a vessel thrown on a wheel, formed by finger pressure in "lifting" the clay
also called throwing marks
rim
the area between the lip or margin and the side wall or neck of a vessel
sometimes used interchangeably with lip, especially if there is no change of orientation between the lip and neck or wall
secondary clay
a clay deposit moved away from the original parent from which it weathered by various natural forces and redeposited
a sedimentary or transported clay, (as distinguished from a primary or residual clay)
sherd (potsherd, shard)
a term archaeologists use to refer to a broken fragment of pottery

shoulder
the upper part of the body of a restricted vessel
that portion between the maximum diameter and the orifice or neck
shrinkage water
the portion of mechanically combined water (WP) that separates the clay particles in a clay/water mass and, when lost during drying, contributes to shrinkage of the body
see also: film water
silicate
a compound in which SiO4 tetrahedrons are major constituents
Clays, for example, are aluminasilicates in which the tetrahedrons are joined primarily as sheets
sinter
a process of adhesion and densification (but not complete fusion or vitrification) of a clay upon heating
close to but below the melting point
this process begins around 800°C when clay particles begin to fuse and spinel minerals form
varies by clay type
slip
a fluid suspension of fine clay and water, used to coat a body before firing or poured into a mold to cast a piece
a non-vitreous coating on a pottery vessel
smectite
a group of clay minerals having a three-layer structure consisting of two silica layers with an alumina layer between, characterized by expandability and relatively high base exchange
Chemical Composition: (OH)4Si8Al4O20 • nH2O
Common smectite clay minerals include montmorillonite, bentonite, and saponite.
soak (soaking period)
the time during which the highest temperature of firing is sustained
i.e. the sustained maximum firing temperature
stoneware
a vitreous or semivitreous ceramic ware, requiring higher temperatures (>1000°C)
usually gray, brown, or white, and frequently glazed
temper
to mix or modify to a proper consistency
a material — mineral or organic, but usually nonplasticadded to a clay to improve its working, drying, or firing properties
see also: additive, aggregate, aplastic, grog, inclusion, nonplastic
terra sigillata
a red-slipped earthenware associated with ancient Greece and Rome
refers to a red or black earthenware slip or "slip glaze" often used for painting elaborate scenes

thermal stress
internal stresses in a ceramic body arising from repeated subjection to temperature changes and the resulting thermal expansion and contraction of the components in the body
thin section
a piece of rock or ceramic, ground to extreme thinness (0.03 mm) and mounted between glass slides, usually for study in a polarizing microscope