CHEM MOD 7
1/184
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
185 Terms
Organic compounds
compounds with carbon atoms covalently bonded to each other and to other non-metallic atoms.
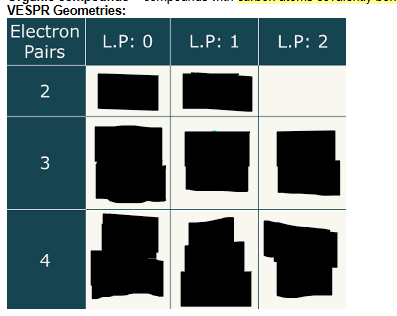
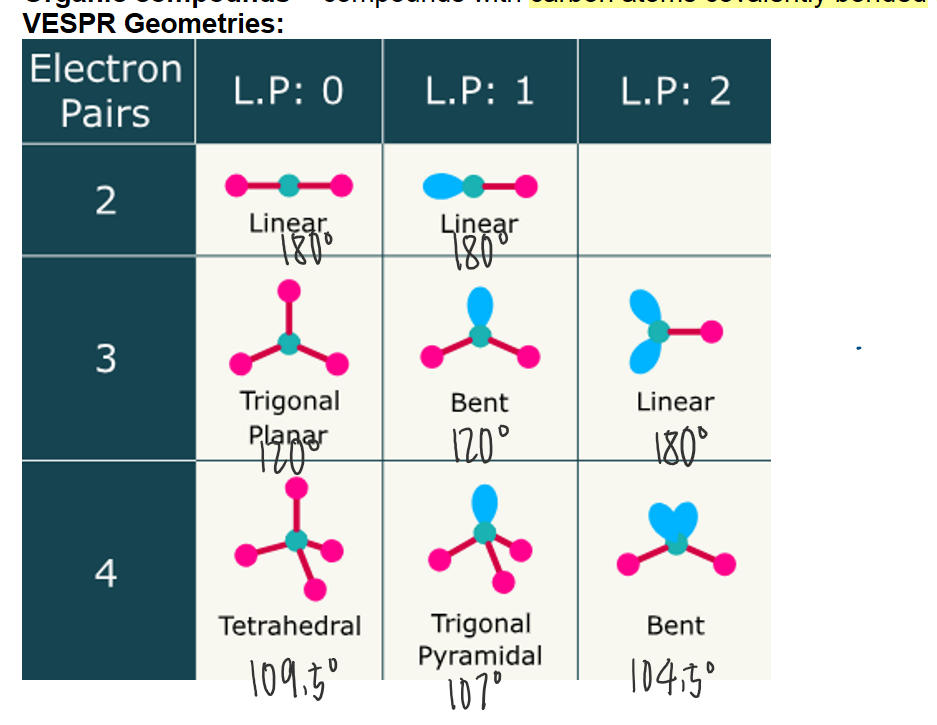
VESPR Geometries
IUPAC
International Union of Pure and Applied Chemistry
An organisation that issues rules for naming chemical compounds, so that all chemists can use the same names.
Naming hydrogen carbons
A stem/parent which indicates the number of carbon atoms that form the longest continuous chain.
A suffix which indicates the family to which the compound belongs
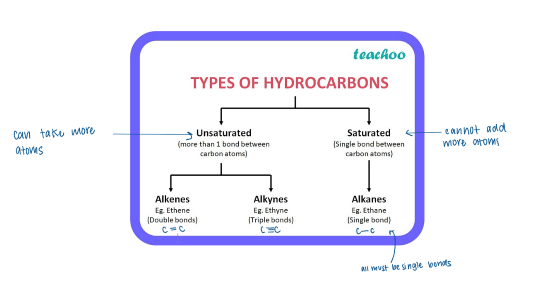
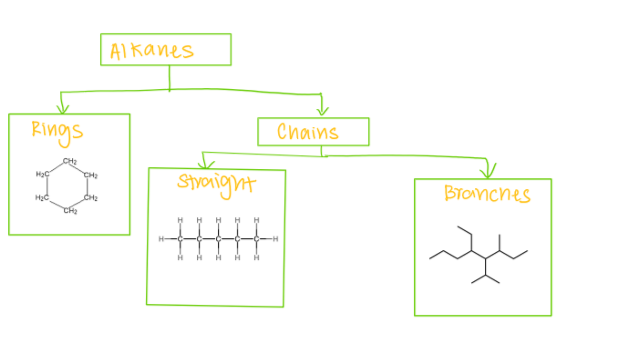
Alkanes
= contains only C-C single bonds in the backbone
Also called saturated hydrocarbons because no more hydrogen atoms can be added to the carbon atoms
Have the same general formula: CnH2n+2
Functional group
an atom or group of atoms which give a compound some characteristic physical and chemical properties.
Alkenes
Contains at least one C=C double bond in backbone
Unsaturated hydrocarbons
General formula: CnH2n
Alkynes
Contain at least one C≡C triple bond in backbone
Unsaturated hydrocarbons
General formula: CnH2n-2
Hydrocarbons
= the simplest organic compounds, composed of only carbons and hydrogen.
Main constituents of natural gas and crude oil.
Types of hydrocarbons
Types of Alkanes
Branched Alkanes
Alkane compounds containing alkyl groups
Alkyl groups have one less hydrogen than the corresponding alkane because they branch off the main chain.
Homologous series + members of the same homologous series have:
A family of hydrocarbons with similar chemical properties who share the same general formula.
Members of the same homologous series have:
A similar structure and the same general formula (each member of a homologous series differ by a -CH2- unit from the previous member.
A pattern to their physical properties
Similar chemical properties.
What determines the intermolecular forces that occur between molecules?
Polarity (polar/nonpolar): type of intermolecular forces
Size of molecular: strength of dispersion force
Packing: geometry
Are hydrocarbons polar or non-polar? explain
Non-polar, although C-H is a polar bond due to molecular shape, overall, it yields a zero vector sum, hence, hydrocarbons are non-polar molecules.
What affects the strength of the intermolecular force between hydrocarbon molecules?
Dispersion forces: size of the molecules (molar mass), packing efficiency
Boiling and melting point
= are measures of the thermal energy required to break intermolecular forces.
Boiling point = The thermal energy needed to cause a transition inside between liquid to gas breaking IMF.
Melting point = The thermal energy needed to cause a transition inside between solid to liquid breaking IMF.
What affects the boiling/melting point for hydrocarbons
Chains with even numbers of carbon atoms pack slightly more efficiently in the solid crystalline state than chains with odd numbers.
Molar mass:
As chain length increase , molar mass increases
The strength of dispersion force will increase
More heat energy is required to break the stronger intermolecular forces and causing boiling/melting point.
Packing:
Smaller or unbranched molecules pack more efficiently.
The strengths of intermolecular forces will increase (decrease in distance)
More heat energy is required to break the stronger intermolecular forces and causing boiling/melting.
The molar mass, polarity, packing strength between alkanes, alkenes, and alkynes. (melting/boiling point effect)
Alkenes have slightly lower boiling points than alkanes,
Alkynes have slightly higher boiling points than alkanes.
Bonds | Molar mass | Polarity | Packing |
Alkane | XXX | X | X |
Alkenes | XX | XX | XX |
Alkynes | X | XXX | XXX |
Solubility
If a substance is soluble:
If a substance is insoluble:
If a substance is soluble, the adhesive forces are strong enough to break the cohesive forces to cause mixing at a molecular level.
If a substance is insoluble, the cohesive forces are too strong to be broken by the adhesive forces.
Solubility of hydrocarbons
Hydrocarbons exhibit dispersion forces as its cohesive intermolecular force, while water exhibits hydrogen bonding as its cohesive intermolecular force. As the adhesive dispersion force interaction between water and hydrocarbons is too weak to disrupt the strong hydrogen bonding in the solvent, hydrocarbons will be insoluble.
Density definition + relation to IMF of hydrocarbons
= A measure of its mass per unit volume.
As molar mass increases there is an increase dispersion force strength, getting more packed as the molecules are attracted.
Volatility definition + relation to IMF of hydrocarbons
= The ability of a liquid to form a vapour. Measured by vapour pressure which is a measure of the concentration in the gas phase above the liquid.
Hydrocarbons have relatively high volatility:
Weak dispersion forces, low IMF, low BP, high BP.
Viscosity definition + relation to IMF of hydrocarbons
= refers to a substance's resistance to fluid flow. Liquids with a relatively high resistance to flow have high viscosity
Viscosity depends on the:
Strength of intermolecular force: High IMF --> High viscosity
Size of molecules: High size --> High viscosity
Larger molecules exhibit stronger dispersion forces and larger molecules tend to become more tangled up, which prevents easy flow.
Temperature: High temp --> low viscosity
Molecules have greater kinetic energy. Molecules move around more which increases the space between molecules. Weaker intermolecular force.
Alcohol
formed when a hydrogen atom on a hydrocarbon has been replaced with a hydroxyl (-OH) group.
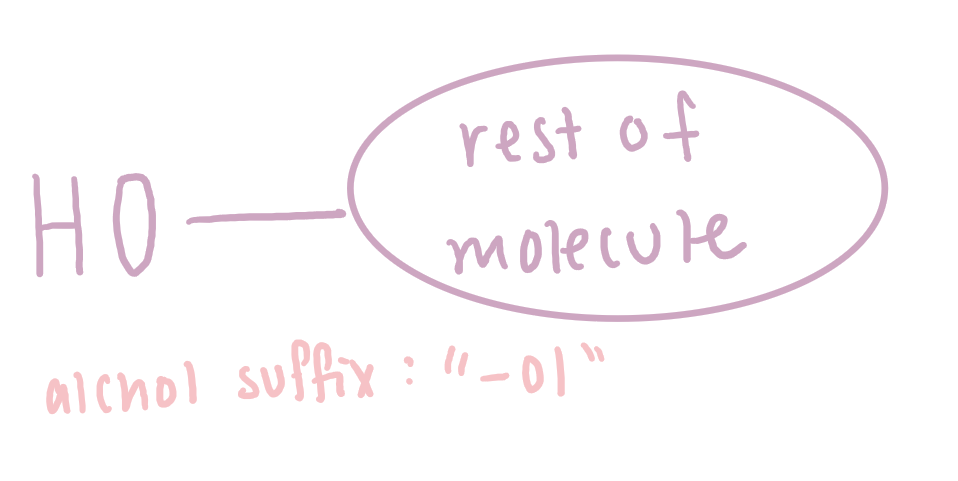
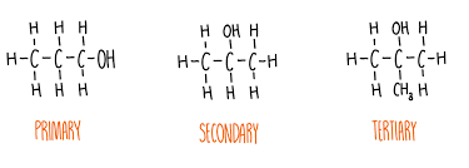
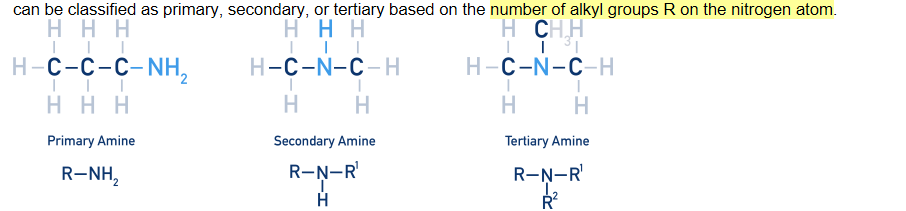
Alcohols can be classified as:
Primary (1°) alcohol have one other carbon atom bonded to the carbon bearing of the -OH group.
Secondary (2°) alcohols have two other carbon atoms bonded to the carbon bearing the -OH group.
Tertiary (3°) alcohols have three other carbon atoms bonded to the carbon bearing the -OH group.
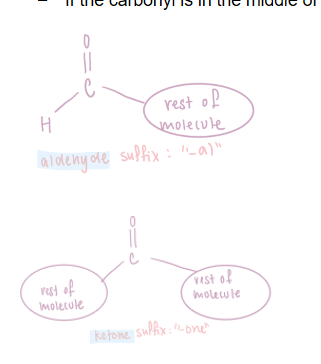
Aldehydes and ketones
Contain the carbonyl functional group, which has a carbon double bonded to an oxygen, C=O.
The difference between aldehydes and ketones is the position of the carbonyl on the carbon chain:
If the carbonyl is on a terminal carbon, it is an aldehyde, named with the suffix "-al".
If the carbonyl is in the middle of the carbon chain it is a ketone, named with the suffix "-one".
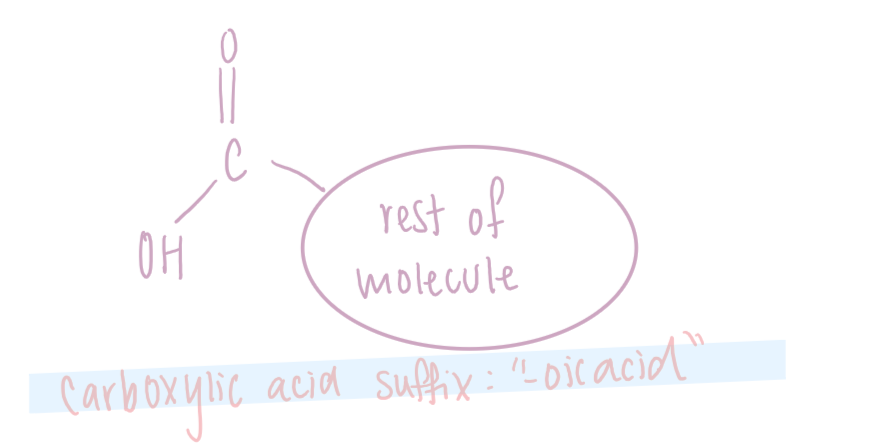
Carboxylic acid
Contains the carboxyl functional group, which has a carbonyl, C=O bonded to a hydroxyl, -OH. The carboxyl group is abbreviated as COOH and has the suffix "-oic acid".
Since the carboxyl group uses up 3 bonds on the carbon atom, it must be on a terminal carbon.
Amines
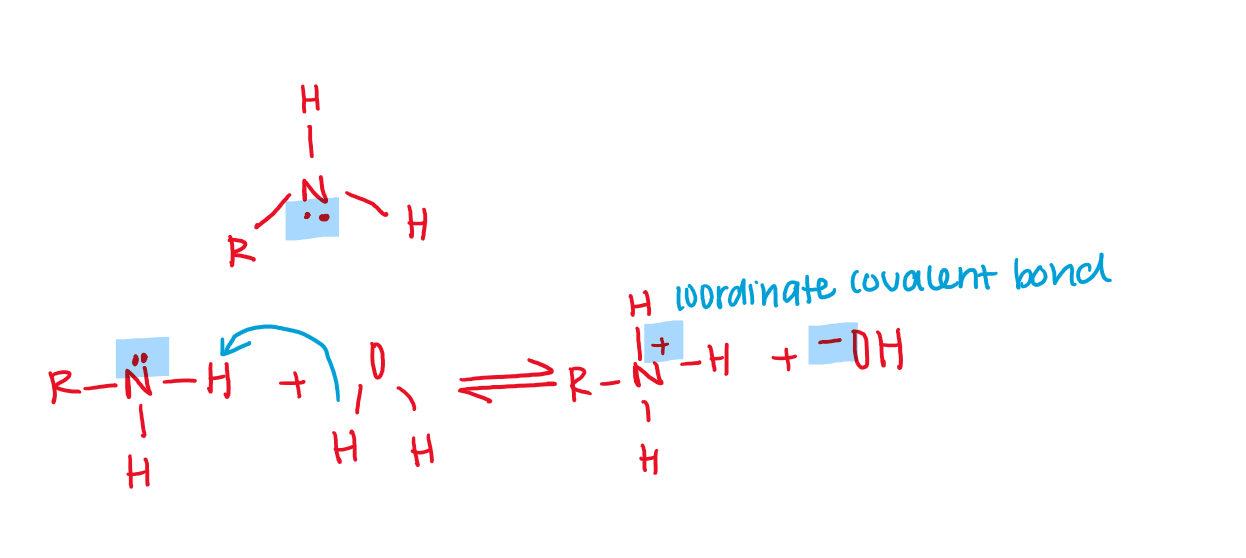
Amines contains an nitrogen with a lone electron pair that can accept H+.
Like with ammonia, the lone pair on the nitrogen atom of an amine can accept a proton to form an ammonium ion.
Amines are weak bases.
How can amines and amides be classified
Halogenated organic compounds
When one or more hydrogen atoms on a hydrocarbon have been replaced with a halogen.
The halogen functional groups are treated as substituents.
The halogen functional groups are named using prefixes placed in front of the name of the hydrocarbon. They do not alter the suffix.
F:fluoro
Cl: chloro
Br: bromo
I: iodo
Special rule: If the numbers are completely identical in both directions, then the name that comes first numerically is correct.
Structural isomers
Are molecules that have the same molecular formula but their atoms are arranged differently to give different structural formulae.
Molecular formula tells us the types of atoms present but not its arrangement while structural formulas reveal more information on how a set of atoms are arranged.
Chain isomers
Involve rearrangement of the carbons in the backbone. There may be a different number of carbons in the longest chain, or different branching in the carbon chain.
Position isomers
Result when molecules have the same carbon skeleton and same functional group but the functional group is at different location.
Functional group isomers
When the atoms in the molecules are arranged to form different functional groups.
Functional groups isomers belong to different homologous series
Geometric isomers
Rotation around a single bond occurs readily, but rotation around a double bond is restricted.
Sp3 hybridization
Alkanes
When the carbon atom is bonded to four other atoms the hybridization is said to be sp3 type. Here 1 s orbital and 3 p orbitals in the same shell of an atom combine to form four new equivalent orbitals. The arrangement is tetrahedral with a bond angle of 109.5o. Example: Hybridization of CH4 (Methane)
SP2 hybridisation
Alkene
A carbon atom is sp2 hybridized when bonding takes place between 1 s-orbital with two p orbitals. There is a formation of two single bonds and one double bond between three atoms. The hybrid orbitals are placed in a triangular planar arrangement with 120° angles between bonds. Example: Hybridization of ethene
sp hybridisation
Alkyne
Carbon can have an sp hybridization when it is bound to two other atoms with the help of two double bonds or one single and one triple bond. When the hybridization occurs the molecules have a linear arrangement of the atoms with a bond angle of 180°. Example: Hybridization of CO2.
Bond rotation of single and double bonds
Alkanes have only single bonds, which allows them to be flexible - this impacts on their shape and hence on their physical properties.
Alkenes have a double bond. The atoms linked by the double C=C bond are fixed in a plane at close to 120o angles. This rigidity is caused by the presence of electron clouds, holding 2 electrons in π bonds above and below the line joining the nuclei of the 2 atoms. There are also 2 electrons in a strong σ bond between the atoms.
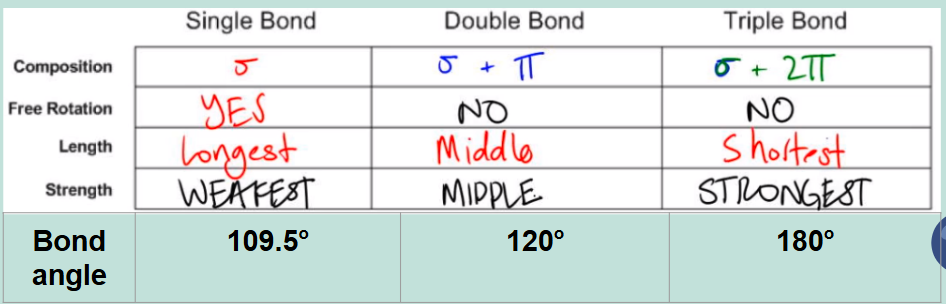
Summary of single, double, triple bonds: Composition, free rotation, length, strength, and bond angle.
Incomplete combustion
occurs when there is insufficient oxygen. The products are water and two unwanted pollutants: soot, C(s) and carbon monoxide, Co(g). They are toxic to humans.
Incomplete combustion produces less energy per mole of fuel combusted, making it less efficient
Alkenes and alkynes tend to combust less completely than alkanes due to the higher carbon content. Some of the carbon may not combine with oxygen.
enthalpy of combustion
energy release from a combustion rxn recorded at standard conditions
why is the enthalpy of combustion more exothermic for successive alkanes?
As chain length increases, the molecule exhibits more C-C and C-H bonds. Hence, in combustion more bonds are being broken and formed releasing a large amount of energy.
reactions of saturated hydrcarbons based on bond energy
Bond energy dictates the reactivity of hydrocarbons compounds:
Subsequent carbon-carbon bonds in a multiple bond are weaker than the original single covalent bond.
Alkenes and alkynes are highly reactive compared to alkanes as double and triple bonds can break open more easily and allow more atoms to join (saturate).
Alkenes and alkynes are able to react in addition reactions.
Alkenes undergo addition reaction with:
Hydrogens (with a catalyst such as Pt, Pd, Ni, or Rh_
Halogens (Cl2 and Br2)
Hydrohalic acids (HF, HCl, HBr, HI)
Water (with a catalyst such as dilute H2SO4)
Hydrogenation
Alkenes react with hydrogen gas (H2) in the presence of certain metal catalysts to produce alkanes.
Appropriate metal catalysts include platinum, palladium, nickel, and rhodium.
MUST BE 180-200 DEGREES
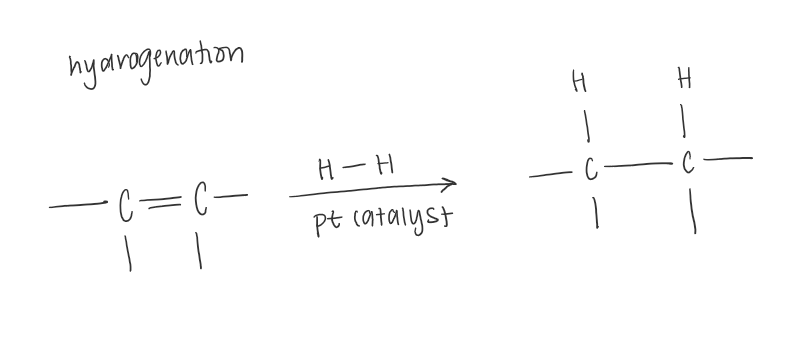
Halogenation (bromination and chlorination)
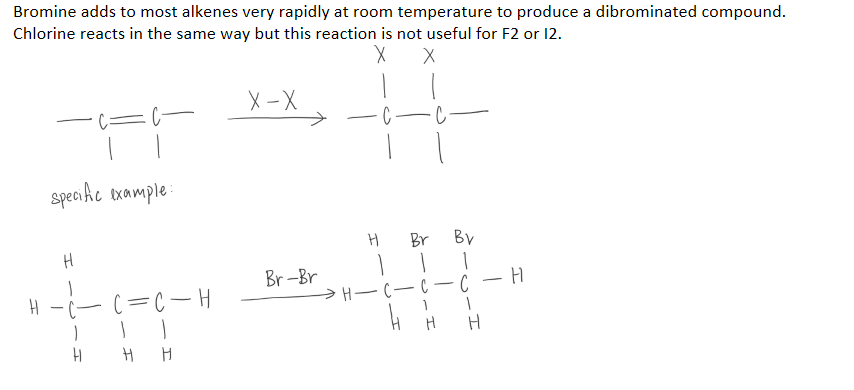
Addition of HX (hydrohalogenation)
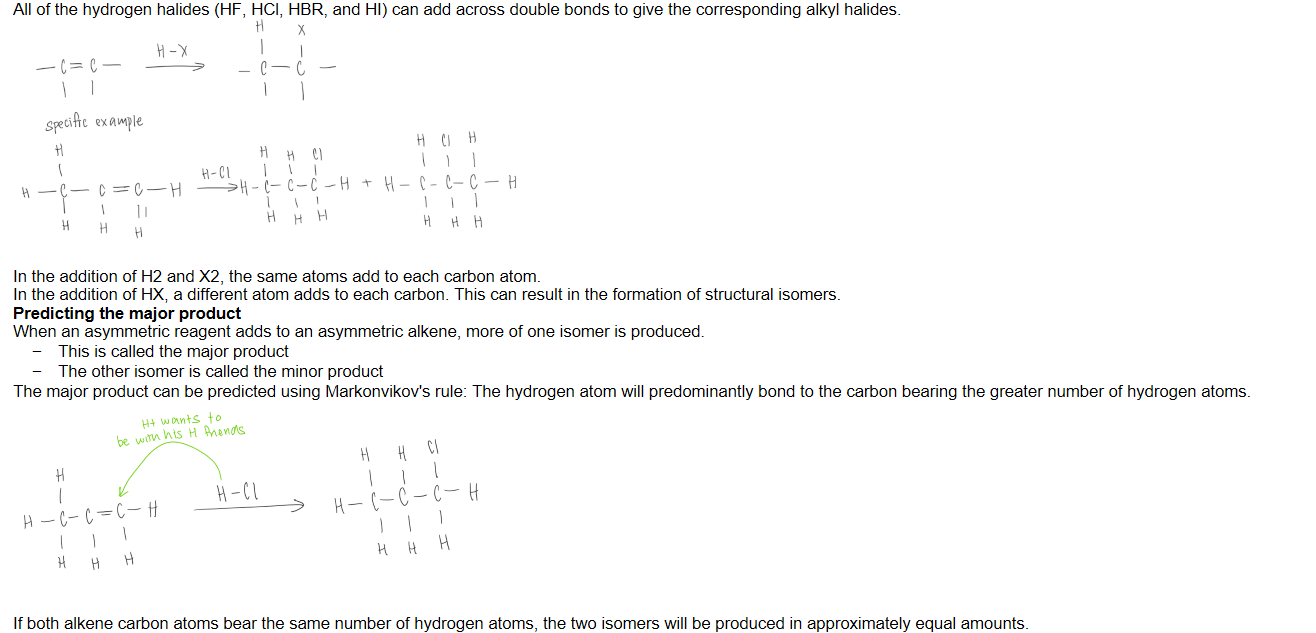
Addition of H20 (hydration)
Substitution reaction of alkanes
First hand investigation: test for unsaturation
Bromine test for unsaturation
The addition of bromine across double and triple bonds can be used as a test for this presence of unsaturation.
If the deep red colour of BR2 disappears, a double or triple bond is present
Although chlorine will also react, it is not commonly used in this test because it is a toxic gas at room temperature, which makes it difficult to handle. Additionally, it is a pale green colour so the results are difficult to distinguish.
Cyclo is more stable thus being more safe.
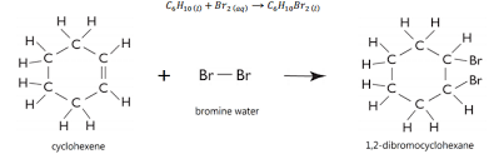
Test for unsaturation: procedure
Place a 3mL of unknown organic liquids (cyclohexane and cyclohexene) in each test tube, labelled A AND b.
Add o.5mL of bromine to each test tube
Record observations.
Br should be minimal to avoid excess.
Safety: bromine test for unsaturation
Many organic compounds are volatile, thus can easily vaporise at room temperature.
The vapours can be toxic or carcinogenic if inhaled or absorbed through skin.
The vapours can also form an explosive mixture with oxygen in the air.
| Bromine | Cyclohexane and cyclohexene |
Hazard | Skin/eye irritant, lung sensitiser | Skin/eye irritant, flammable, toxic by inhalation or ingestion |
Risk | Can cause sever eye damage and skin rashes. If inhaled, can lead to breathing difficulties | Exposure may lead to headaches, dizziness, nausea, and vomiting. Can cause ignition |
Safety precaution | PPE | Fume cupboard, use small quantities, PPE |
Validity: bromine test for unsaturation
Control variables: amount of BR, amount of unknown alkene, alkane, absent of UV light
To improve validity: conduct experiment in dark room or cover test tube in foil to remove UV light
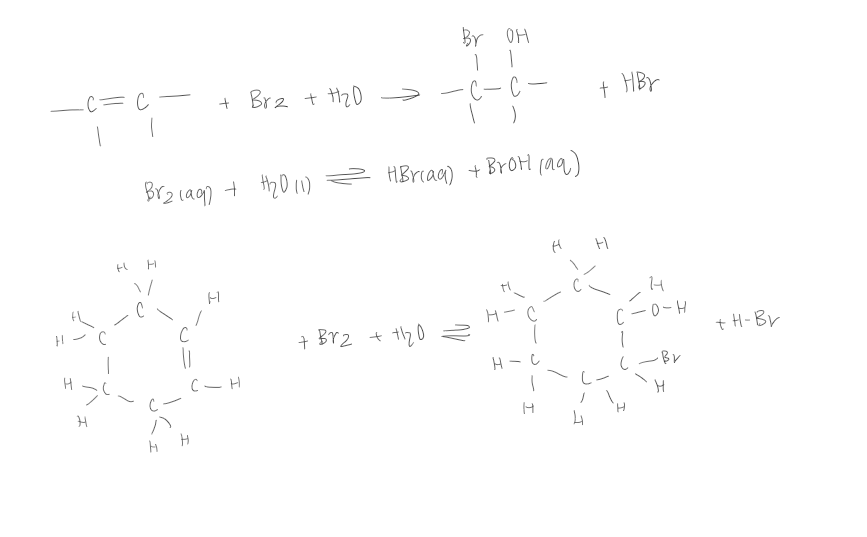
Test for unsaturation: What happens when Bromine water is used instead of liquid bromine
A positive test will still result in the decolourisation of the bromine water
However, water will also react to produce a bromohydrin, containing a bromine atom and a hydroxyl group
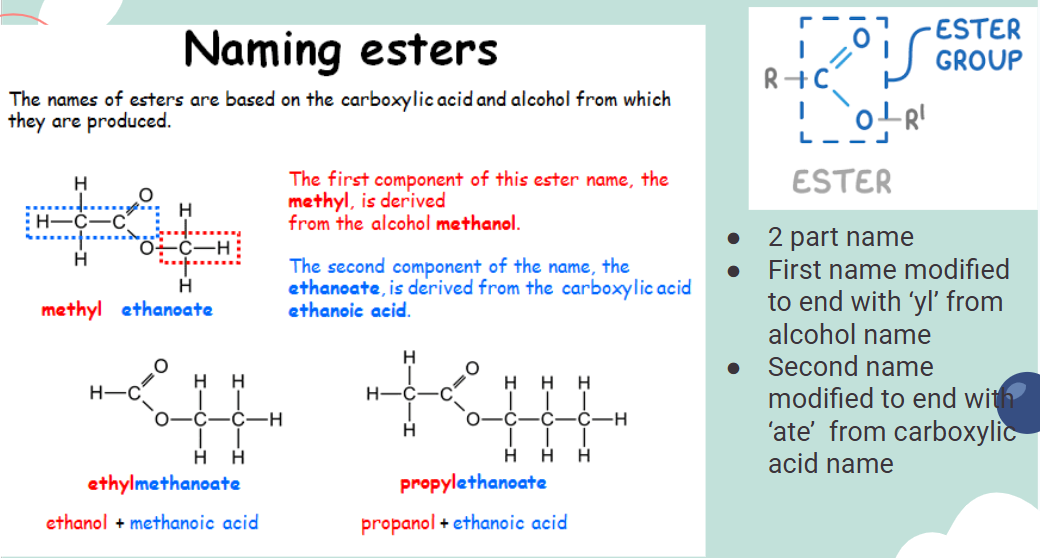
Naming esters
MP of propane
is the lowest due to the molecule forming a more spherical shape in its crystalline form which results in poorest packing and weakest IMF.
BP vs. MP pattern in straight-chain alkanes
The BP increases in a regular pattern with increasing carbon chain length.
Although the MP generally increases with increasing chain length, the MP change is not regular due to the less efficient packing in odd numbered carbon chains compared to even numbered carbon chains, requiring lesser amount of energy to overcome intermolecular dispersion forces.
Haloalkane MP/BP pattern
Haloalkanes have higher melting points that the corresponding hydrocarbons.
This is due to stronger dipole-dipole forces between haloalkane molecules in addiction to dispersion forces caused by the presence of polar halogen-carbon bonds, which make the molecules polar.
How are hydrocarbons extracted from the Earth?
Locate reservoirs via seismic surveys (e.g., underground explosions to map geology).
Drill wells into oil reservoirs to extract crude oil (mainly alkanes).
Fractionally distill crude oil to separate hydrocarbons by boiling point/chain length.
Crack large molecules into smaller, more useful fractions.
Common use of alkanes
Fuels – methane (natural gas), propane and butane in LPG,octane in petrol, longer chain alkanes in aviation fuel and diesel.
Solvents – pentane and hexane.
Waxes & bitumen - Long-chain alkanes
Common uses of alkenes
Used extensively in the chemical industry, due to the reactivity of the C=C bond.
Ethylene can be converted into ethanol, haloalkanes, addition polymers, etc.
Polymers such as polyethylene, polystyrene and PVC are derivatives of ethylene which are used for construction, insulation, toys, food wraps, etc.
Common uses of alkynes
Ethyne (preferred name acetylene) is used as a fuel for welding.
Used in chemical syntheses, as they all contain the reactive C–C triple bond.
Many modern pharmaceuticals are produced from alkynes.
Risks involved in the handling, using and disposing of organic substances such as hydrocarbons.
Volatile & Combustible: Hydrocarbons easily vaporise and catch fire, so avoid naked flames and use a fume cabinet.
Health Hazards: Vapours can be toxic—may be absorbed through skin or lungs. Handle with gloves and in well-ventilated areas.
Incomplete Combustion: Produces carbon monoxide (poisonous) and soot (respiratory issues, pollution).
Disposal: Due to toxicity, hydrocarbons must be collected for safe disposal, not poured down the sink.
solubility of haloalkanes in water
Haloalkanes are slightly more soluble than hydrocarbons in water due to the polarity of the carbon-halogen bond, allowing for dipole-dipole interactions with water.
However, the solubility is still very low because the overall influence of the dipole–dipole interaction is small.
The influence of the halogen decreases as the length of the carbon chain increases.
What can hydrocarbons be miscible in?
In non-polar solvents such as hexane, toluene, tetrachloroethane, white spirit (mixture of alkanes).
Markonikov’s rule
The hydrogen atom will predominantly bond to the carbon atom bearing the greatest number of hydrogen atoms.
WHy must UV light be excluded from bromine, hexane/hexene experiment
Exposure to UV light will cause a substitution reaction to occur between an alkane and BR2. This rxn will cause the bromine to decolourise rending the experiment invalid.
Environmental implication of hydrocarbons
✅ Positive: Hydrocarbons (e.g. natural gas) are used in energy-efficient fuels, reducing reliance on dirtier energy sources like coal.
❌ Negative: Hydrocarbon combustion produces greenhouse gases and pollutants (e.g. CO₂, soot), contributing to climate change and air pollution.
Sociocultural Implications of Hydrocarbons
75% of Australian households powered by hydrocarbon coal irritant.
✅ Positive: Access to hydrocarbon energy supports modern lifestyles, transportation, and infrastructure development in many societies.
❌ Negative: Oil spills and pollution from hydrocarbon use can damage communities, especially Indigenous groups and coastal populations
Economic Implications of Hydrocarbons
✅ Positive: The hydrocarbon industry creates jobs and is a major contributor to national economies through exports and energy production.
❌ Negative: Economic dependence on hydrocarbons can be risky due to fluctuating oil prices and costs of environmental damage or cleanup.
I.e. 1973 oil crisis - led to drastic spike in oil prices: Iran vs. U.S. —> geopolitical instability
Substitution reactions of haloalkanes
Chloroalkanes, bromoalkanes, and iodoalkanes react with aqueous sodium or potassium hydroxide to form alkanois. This is a substiution reaction. The haloalkane is heated under reflux with the hydroxide solution.
BP of alcohols
Alcohols exhibit both polar and non-polar regions.
As the chain length increases within a homologous series the non-polar region increases in size, as a result dispersion size strength increases.
More thermal energy is needed to break stronger intermolecular forces and cause a transition in state.
Thus the trend we observe is a linear relationship between molar mass and boiling point within a homologous series.
MP of alcohols
Proportional relationship between MM and melting point, however, packing efficiency has a greater effect for substances in a solid state, hence, slight deviation exist in this trend.
Ethanol as a solvent
Polar
Ethanol is a good solvent because it is a polar molecule
In both pure water and pure ethanol the main intermolecular attractions are hydrogen bonds.
When ethanol and water are mixed they readily dissolved in each due to the polar nature of the O-H bond.
Main interactions as a polar solvent: dipole-dipole and hydrogen.
Non-polar
Ethanol and hexane (a non-polar molecule) readily dissolves in each other.
Main interaction as non-polar solvent: dispersion forces.
Water Solubility of alcohols
The solubility of alcohols in water is dictated by size because of the opposing effects of the polar and non-polar portions of the molecule.
The polar hydroxyl group is hydrophillic and the non-polar hydrocarbon chain is hydrophobic.
Water solubility decreases with increasing chain length as water cannot solvate large non-polar carbon chains.
The -OH group can form hydrogen bonds with water
However to solvate the large non-polar chain, many strong cohesive hydrogen bonds between water molecule needs to broken. These cannot be broken by weak adhesive dispersion forces.
Lower solubility of the bigger alcohols
With an increase in molecular mass, the non-polar alkyle group (HC chain) becomes predominant and masks the effect of polar -OH group.
Although the polar -OH end of the alcohol molecules can form new hydrogen bonds with water molecules, the longer and more significant non polar HC “tail” doesn’t form hydrogen bonds and gets in the way of the interaction between the polar ends of the alcohol and water molecules.
Solubility of isometric alcohols
In isometric alcohols, branching generally increases solubility in water, while longer straight-chain isomers are less soluble. This is because branching reduces the surface area of the non-polar hydrocarbon portion, minimizing its repulsion with water.
primary, secondary, and tertiary BP
Secondary and tertiary alcohols have lower boiling points than primary alcohols with the same number of carbon atoms.
the three alcohols are isomers so their dispersion forces will be the same.
However, in secondary and tertiary alcohols there are alkyl groups adjacent to the OH. These hinder the OH groups from getting close together and restricts the formation of strong hydrogen bonds.
Primary> secondary> teritary
Alcohol solubility in non-polar organic solvents
Become more soluble as the length of the carbon chain increases.
The large alkyl chain can form strong dispersion forces with other non-polar substances. These are strong enough to disrupt the cohesive hydrogen bonds in water.
Large alcohols solubility
Large alcohols = multiple hydroxyl groups
Can be soluble in water as they form strong adhesive hydrogen bonds with water.
Small alcohol as solvents
Are good solvents for dissolving both polar and non-polar substances.
The polar hydroxyl group can form polar interactions such as ion-dipole forces, hydrogen bonding and dipole-dipole forces with polar and ionic substances.
The non-polar hydrocarbon chain can form dispersion forces with non-polar substances.
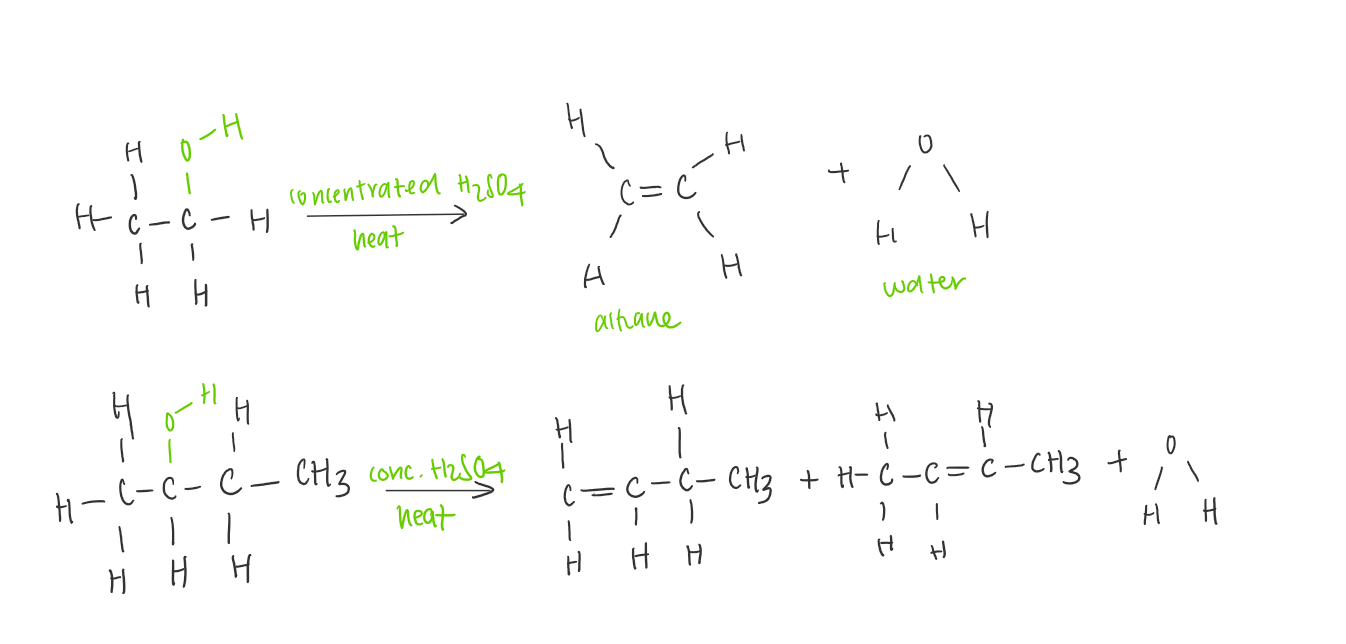
Dehydration of alcohols
When alcohols are heated with concentrated sulfuric or phosphoric acid as a catalyst, an OH group and H atom on the adjacent carbon will be eliminated to give an alkene and water.
This is classified as an elimination rxn
When assymetric secondary and tertiary alcohols are dehydrated, a mixture of alkenes will be formed.
Dehydration/substitution: reactivity and ROR
Tertiary alcohol are the most reactive and always react the fastest. Dehydration occurs readily at room temperature
Primary and secondary alcohols require higher temperatures.
Reactivity increases primary < secondary< tertiary
Substitution of alcohols
Occurs when an atom or functional group in a molecule is replaced or “substituted” by another atom or group.
Alcoholds undergo substitution with hydrogen halides (HCl, HBr or HI) to given an alkyl halide and water.
C-O has a lower bond energy than C-H, therefore alcohols undergo substitution more readily than alkanes.
Catalyst = ZNCl2
ROR based on the hydrogen halide in substitution of alcohols
Reactivity increases as you go down the halogen group
HCl< HBr< HI
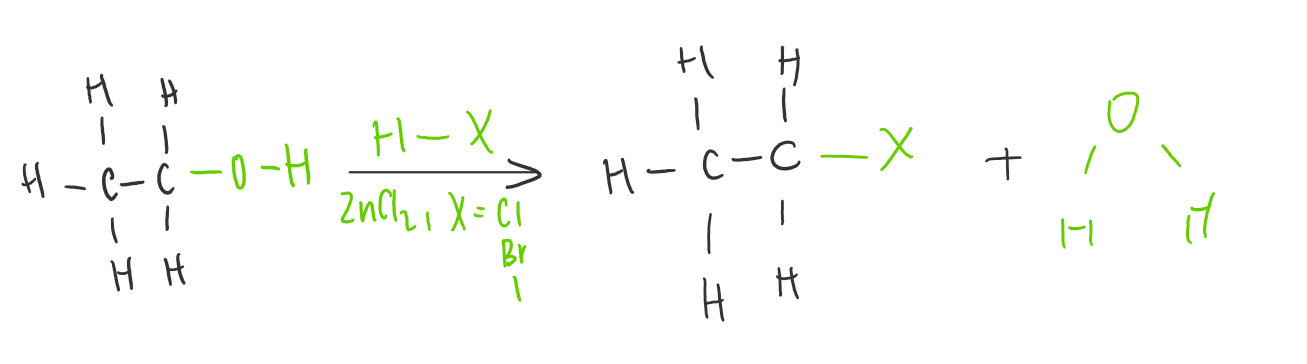
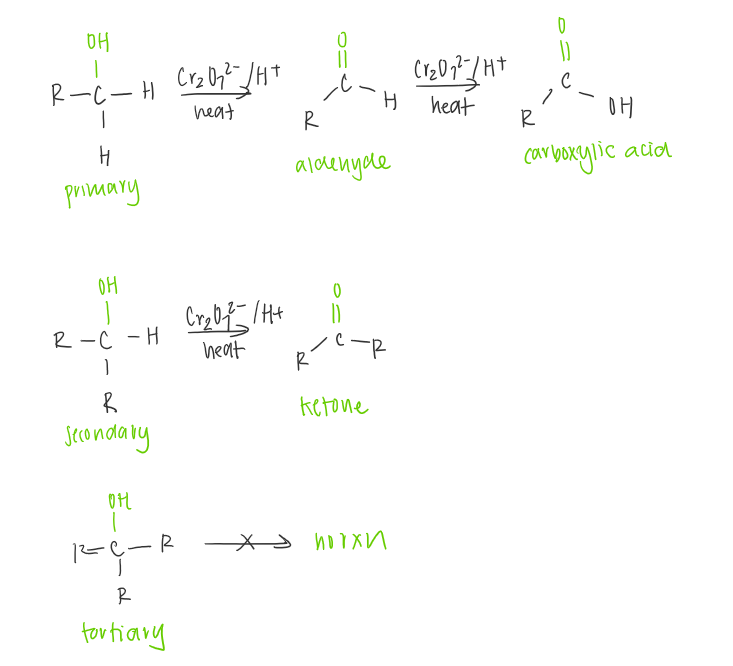
Oxidation of alcohols
Alcohols can be oxidised with strong oxiding agents to give carbonyl compounds.
The oxidising agent commonly used are acidic permanganate (MnO4-) or dichromate (Cr2072-) solutions.
It can be seen in the change in the oxidation state for the carbon atoms. The increase in oxidation state of the carbon atom means that electrons have been lost from the carbon atom.
Primary
alcohol —> aldehyde —> carboxylic acid
Secondary
alcohol —> ketone
tertiary
no rxn as the alcohol carbon has no hydrogen atoms
Oxidation states in organic molecules
is assigned based on the electronegativities of the bonded atoms.
Identification of types of alcohols oxidation
MnO4- —> Mn2+ (purple —> colourless)
Cr2O72- —> Cr3+ (orange —> green)
Colour change in increasing order: tertiary<secondary<primary

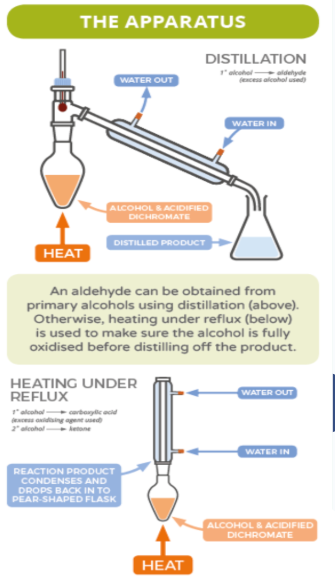
How to stop primary alcohol oxidation at aldehyde?
The oxidising agent can be slowly added to a heated solution of the alcohol and the aldehyde is quickly distilled off.
alcohol combustion - why are they good fuels?
alcohols are good fuels as the combustion of alcohols is highly exothermic. since they are are already oxygenated, alcohols are more prone to complete combustion
investigation: enthalpy of combustion
Different fuels produce different amount of heat. To compare different alcohols as fuels, we can use the standard enthalpy of combustion
calorimetry
Instrument used to measure temp change in combustion rxn through which enthalpy of combustion can be calculated
experimental procedure - enthalpy of combustion
Add 200ml water to the copper can. record the initial temperature
clamp the can above the spirit burner so that the tip of the flam will just touch the can when lit
Record the mass of the spirit burner with the cap on, place the burner under the can.
Light the wick and gently stir the water with a stirring rod.
When the temperature has risen by above 15degrees, extinguish the flame by replacing the cap
Accurately record the maximum temperature of the water
Reweigh the burner and record its final mass
Repeat with a different fuel
Safety for enthalpy of combustion
Methanol - toxic in all routes of expose, permanent blindness if ingested.
Ethanol - highly flammable, slightly toxic if ingested
Propan-1-ol - highly flammable, toxic if ingested or inhaled
alcohol molecular formula general
CNH2N+1OH
enthalpy of combustion accuracy, validity, reliability
Accuracy:
Parallax error - measuring cylinder + thermometer
Calibration - scale balance
Assumption of water density being 1g/L
Validity: Considered invalid as
the method cannot achieve the desired outcome - to determine the heat of combustion of the fuel.
Heat loss to surroundings
Incomplete combustion - soot formed on the bottom of the beaker
Reliable:
repeated trials
How to improve validity of results - Heat of combustion (alcohol)
Insulated containers
Stop air currents around the experiment
Reduce the distance between the flame and the Beaker.
Stir water.
Bomb calorimeter
Lucas test
Used to differentiate between the types of alcohols based on the difference in their reactivity. a solution of anhydrous zinc chloride in concentrated hydrochloric acid.
A positive test is indicated by an increase in the turbidity or cloudiness of the rxn mixture and the formation of two immiscible layers.
1 = no rxn
2 = slow, slightly cloudy
3 = fast, cloudy
